Tutorial 4: Instrumental Variables#
Week 3, Day 5: Network Causality
By Neuromatch Academy
Content creators: Ari Benjamin, Tony Liu, Konrad Kording
Content reviewers: Mike X Cohen, Madineh Sarvestani, Yoni Friedman, Ella Batty, Michael Waskom
Production editors: Gagana B, Spiros Chavlis
Tutorial objectives#
Estimated timing of tutorial: 1 hour, 5 min
This is our final tutorial on our day of examining causality. Below is the high level outline of what we’ve covered today, with the sections we will focus on in this notebook in bold:
Master definitions of causality
Understand that estimating causality is possible
Learn 4 different methods and understand when they fail
perturbations
correlations
simultaneous fitting/regression
instrumental variables
Tutorial 4 Objectives
In tutorial 3 we saw that even more sophisticated techniques such as simultaneous fitting fail to capture causality in the presence of omitted variable bias. So what techniques are there for us to obtain valid causal measurements when we can’t perturb the system? Here we will:
learn about instrumental variables, a method that does not require experimental data for valid causal analysis
explore benefits of instrumental variable analysis and limitations
addresses omitted variable bias seen in regression
less efficient in terms of sample size than other techniques
requires a particular form of randomness in the system in order for causal effects to be identified
Setup#
Install and import feedback gadget#
Show code cell source
# @title Install and import feedback gadget
!pip3 install vibecheck datatops --quiet
from vibecheck import DatatopsContentReviewContainer
def content_review(notebook_section: str):
return DatatopsContentReviewContainer(
"", # No text prompt
notebook_section,
{
"url": "https://pmyvdlilci.execute-api.us-east-1.amazonaws.com/klab",
"name": "neuromatch_cn",
"user_key": "y1x3mpx5",
},
).render()
feedback_prefix = "W3D5_T4"
# Imports
import numpy as np
import matplotlib.pyplot as plt
from mpl_toolkits.axes_grid1 import make_axes_locatable
from sklearn.multioutput import MultiOutputRegressor
from sklearn.linear_model import LinearRegression, Lasso
Figure Settings#
Show code cell source
# @title Figure Settings
import logging
logging.getLogger('matplotlib.font_manager').disabled = True
import ipywidgets as widgets # interactive display
%config InlineBackend.figure_format = 'retina'
plt.style.use("https://raw.githubusercontent.com/NeuromatchAcademy/course-content/main/nma.mplstyle")
Plotting Functions#
Show code cell source
# @title Plotting Functions
def see_neurons(A, ax, show=False):
"""
Visualizes the connectivity matrix.
Args:
A (np.ndarray): the connectivity matrix of shape (n_neurons, n_neurons)
ax (plt.axis): the matplotlib axis to display on
Returns:
Nothing, but visualizes A.
"""
A = A.T # make up for opposite connectivity
n = len(A)
ax.set_aspect('equal')
thetas = np.linspace(0, np.pi * 2, n,endpoint=False)
x, y = np.cos(thetas), np.sin(thetas),
ax.scatter(x, y, c='k',s=150)
A = A / A.max()
for i in range(n):
for j in range(n):
if A[i, j] > 0:
ax.arrow(x[i], y[i], x[j] - x[i], y[j] - y[i], color='k', alpha=A[i, j], head_width=.15,
width = A[i,j] / 25, shape='right', length_includes_head=True)
ax.axis('off')
if show:
plt.show()
def plot_neural_activity(X):
"""Plot first 10 timesteps of neural activity
Args:
X (ndarray): neural activity (n_neurons by timesteps)
"""
f, ax = plt.subplots()
im = ax.imshow(X[:, :10], aspect='auto')
divider = make_axes_locatable(ax)
cax1 = divider.append_axes("right", size="5%", pad=0.15)
plt.colorbar(im, cax=cax1)
ax.set(xlabel='Timestep', ylabel='Neuron', title='Simulated Neural Activity')
plt.show()
def compare_granger_connectivity(A, reject_null, selected_neuron):
"""Plot granger connectivity vs true
Args:
A (ndarray): true connectivity (n_neurons by n_neurons)
reject_null (list): outcome of granger causality, length n_neurons
selecte_neuron (int): the neuron we are plotting connectivity from
"""
fig, axs = plt.subplots(1, 2, figsize=(10, 5))
im = axs[0].imshow(A[:, [selected_neuron]], cmap='coolwarm', aspect='auto')
plt.colorbar(im, ax=axs[0])
axs[0].set_xticks([0])
axs[0].set_xticklabels([f"Neuron {selected_neuron}"])
axs[0].set_title(f"True connectivity")
im = axs[1].imshow(np.array([reject_null]).transpose(),
cmap='coolwarm', aspect='auto')
plt.colorbar(im, ax=axs[1])
axs[1].set_xticks([0])
axs[1].set_xticklabels([f"Neuron {selected_neuron}"])
axs[1].set_title(f"Granger causality connectivity")
plt.show()
def plot_performance_vs_eta(etas, corr_data):
""" Plot IV estimation performance as a function of instrument strength
Args:
etas (list): list of instrument strengths
corr_data (ndarray): n_trials x len(etas) array where each element is the correlation
between true and estimated connectivity matries for that trial and
instrument strength
"""
corr_mean = corr_data.mean(axis=0)
corr_std = corr_data.std(axis=0)
plt.plot(etas, corr_mean)
plt.fill_between(etas, corr_mean - corr_std, corr_mean + corr_std, alpha=.2)
plt.xlim([etas[0], etas[-1]])
plt.title("IV performance as a function of instrument strength")
plt.ylabel("Correlation b.t. IV and true connectivity")
plt.xlabel("Strength of instrument (eta)")
plt.show()
Helper Functions#
Show code cell source
# @title Helper Functions
def sigmoid(x):
"""
Compute sigmoid nonlinearity element-wise on x.
Args:
x (np.ndarray): the numpy data array we want to transform
Returns
(np.ndarray): x with sigmoid nonlinearity applied
"""
return 1 / (1 + np.exp(-x))
def logit(x):
"""
Applies the logit (inverse sigmoid) transformation
Args:
x (np.ndarray): the numpy data array we want to transform
Returns
(np.ndarray): x with logit nonlinearity applied
"""
return np.log(x/(1-x))
def create_connectivity(n_neurons, random_state=42, p=0.9):
"""
Generate our nxn causal connectivity matrix.
Args:
n_neurons (int): the number of neurons in our system.
random_state (int): random seed for reproducibility
Returns:
A (np.ndarray): our 0.1 sparse connectivity matrix
"""
np.random.seed(random_state)
A_0 = np.random.choice([0, 1], size=(n_neurons, n_neurons), p=[p, 1 - p])
# set the timescale of the dynamical system to about 100 steps
_, s_vals, _ = np.linalg.svd(A_0)
A = A_0 / (1.01 * s_vals[0])
# _, s_val_test, _ = np.linalg.svd(A)
# assert s_val_test[0] < 1, "largest singular value >= 1"
return A
def simulate_neurons(A, timesteps, random_state=42):
"""
Simulates a dynamical system for the specified number of neurons and timesteps.
Args:
A (np.array): the connectivity matrix
timesteps (int): the number of timesteps to simulate our system.
random_state (int): random seed for reproducibility
Returns:
- X has shape (n_neurons, timeteps).
"""
np.random.seed(random_state)
n_neurons = len(A)
X = np.zeros((n_neurons, timesteps))
for t in range(timesteps - 1):
# solution
epsilon = np.random.multivariate_normal(np.zeros(n_neurons), np.eye(n_neurons))
X[:, t + 1] = sigmoid(A.dot(X[:, t]) + epsilon)
assert epsilon.shape == (n_neurons,)
return X
def get_sys_corr(n_neurons, timesteps, random_state=42, neuron_idx=None):
"""
A wrapper function for our correlation calculations between A and R.
Args:
n_neurons (int): the number of neurons in our system.
timesteps (int): the number of timesteps to simulate our system.
random_state (int): seed for reproducibility
neuron_idx (int): optionally provide a neuron idx to slice out
Returns:
A single float correlation value representing the similarity between A and R
"""
A = create_connectivity(n_neurons, random_state)
X = simulate_neurons(A, timesteps)
R = correlation_for_all_neurons(X)
return np.corrcoef(A.flatten(), R.flatten())[0, 1]
def correlation_for_all_neurons(X):
"""Computes the connectivity matrix for the all neurons using correlations
Args:
X: the matrix of activities
Returns:
estimated_connectivity (np.ndarray): estimated connectivity for the selected neuron, of shape (n_neurons,)
"""
n_neurons = len(X)
S = np.concatenate([X[:, 1:], X[:, :-1]], axis=0)
R = np.corrcoef(S)[:n_neurons, n_neurons:]
return R
def print_corr(v1, v2, corrs, idx_dict):
"""Helper function for formatting print statements for correlations"""
text_dict = {'Z':'taxes', 'T':'# cigarettes', 'C':'SES status', 'Y':'birth weight'}
print("Correlation between {} and {} ({} and {}): {:.3f}".format(v1, v2, text_dict[v1], text_dict[v2], corrs[idx_dict[v1], idx_dict[v2]]))
def get_regression_estimate(X, neuron_idx=None):
"""
Estimates the connectivity matrix using lasso regression.
Args:
X (np.ndarray): our simulated system of shape (n_neurons, timesteps)
neuron_idx (int): optionally provide a neuron idx to compute connectivity for
Returns:
V (np.ndarray): estimated connectivity matrix of shape (n_neurons, n_neurons).
if neuron_idx is specified, V is of shape (n_neurons,).
"""
n_neurons = X.shape[0]
# Extract Y and W as defined above
W = X[:, :-1].transpose()
if neuron_idx is None:
Y = X[:, 1:].transpose()
else:
Y = X[[neuron_idx], 1:].transpose()
# apply inverse sigmoid transformation
Y = logit(Y)
# fit multioutput regression
regression = MultiOutputRegressor(Lasso(fit_intercept=False, alpha=0.01), n_jobs=-1)
regression.fit(W,Y)
if neuron_idx is None:
V = np.zeros((n_neurons, n_neurons))
for i, estimator in enumerate(regression.estimators_):
V[i, :] = estimator.coef_
else:
V = regression.estimators_[0].coef_
return V
def get_regression_corr(n_neurons, timesteps, random_state, observed_ratio, regression_args, neuron_idx=None):
"""
A wrapper function for our correlation calculations between A and the V estimated
from regression.
Args:
n_neurons (int): the number of neurons in our system.
timesteps (int): the number of timesteps to simulate our system.
random_state (int): seed for reproducibility
observed_ratio (float): the proportion of n_neurons observed, must be between 0 and 1.
regression_args (dict): dictionary of lasso regression arguments and hyperparameters
neuron_idx (int): optionally provide a neuron idx to compute connectivity for
Returns:
A single float correlation value representing the similarity between A and R
"""
assert (observed_ratio > 0) and (observed_ratio <= 1)
A = create_connectivity(n_neurons, random_state)
X = simulate_neurons(A, timesteps)
sel_idx = np.clip(int(n_neurons*observed_ratio), 1, n_neurons)
sel_X = X[:sel_idx, :]
sel_A = A[:sel_idx, :sel_idx]
sel_V = get_regression_estimate(sel_X, neuron_idx=neuron_idx)
if neuron_idx is None:
return np.corrcoef(sel_A.flatten(), sel_V.flatten())[1, 0]
else:
return np.corrcoef(sel_A[neuron_idx, :], sel_V)[1, 0]
def get_regression_estimate_full_connectivity(X):
"""
Estimates the connectivity matrix using lasso regression.
Args:
X (np.ndarray): our simulated system of shape (n_neurons, timesteps)
neuron_idx (int): optionally provide a neuron idx to compute connectivity for
Returns:
V (np.ndarray): estimated connectivity matrix of shape (n_neurons, n_neurons).
if neuron_idx is specified, V is of shape (n_neurons,).
"""
n_neurons = X.shape[0]
# Extract Y and W as defined above
W = X[:, :-1].transpose()
Y = X[:, 1:].transpose()
# apply inverse sigmoid transformation
Y = logit(Y)
# fit multioutput regression
reg = MultiOutputRegressor(Lasso(fit_intercept=False, alpha=0.01, max_iter=200), n_jobs=-1)
reg.fit(W, Y)
V = np.zeros((n_neurons, n_neurons))
for i, estimator in enumerate(reg.estimators_):
V[i, :] = estimator.coef_
return V
def get_regression_corr_full_connectivity(n_neurons, A, X, observed_ratio, regression_args):
"""
A wrapper function for our correlation calculations between A and the V estimated
from regression.
Args:
n_neurons (int): number of neurons
A (np.ndarray): connectivity matrix
X (np.ndarray): dynamical system
observed_ratio (float): the proportion of n_neurons observed, must be between 0 and 1.
regression_args (dict): dictionary of lasso regression arguments and hyperparameters
Returns:
A single float correlation value representing the similarity between A and R
"""
assert (observed_ratio > 0) and (observed_ratio <= 1)
sel_idx = np.clip(int(n_neurons*observed_ratio), 1, n_neurons)
sel_X = X[:sel_idx, :]
sel_A = A[:sel_idx, :sel_idx]
sel_V = get_regression_estimate_full_connectivity(sel_X)
return np.corrcoef(sel_A.flatten(), sel_V.flatten())[1,0], sel_V
The helper functions defined above are:
sigmoid: computes sigmoid nonlinearity element-wise on input, from Tutorial 1logit: applies the logit (inverse sigmoid) transformation, from Tutorial 3create_connectivity: generates nxn causal connectivity matrix., from Tutorial 1simulate_neurons: simulates a dynamical system for the specified number of neurons and timesteps, from Tutorial 1get_sys_corr: a wrapper function for correlation calculations between A and R, from Tutorial 2correlation_for_all_neurons: computes the connectivity matrix for the all neurons using correlations, from Tutorial 2print_corr: formats print statements for correlationsget_regression_estimate: estimates the connectivity matrix using lasso regression, from Tutorial 3get_regression_corr: a wrapper function for our correlation calculations between A and the V estimated from regression.get_regression_estimate_full_connectivity: estimates the connectivity matrix using lasso regression, from Tutorial 3get_regression_corr_full_connectivity: a wrapper function for our correlation calculations between A and the V estimated from regression, from Tutorial 3
Section 1: Instrumental Variables#
Video 1: Instrumental Variables#
Submit your feedback#
Show code cell source
# @title Submit your feedback
content_review(f"{feedback_prefix}_Instrumental_Variables_Video")
If there is randomness naturally occurring in the system that we can observe, this in effect becomes the perturbations we can use to recover causal effects. This is called an instrumental variable. At high level, an instrumental variable must
Be observable
Affect a covariate you care about
Not affect the outcome, except through the covariate
It’s rare to find these things in the wild, but when you do it’s very powerful.
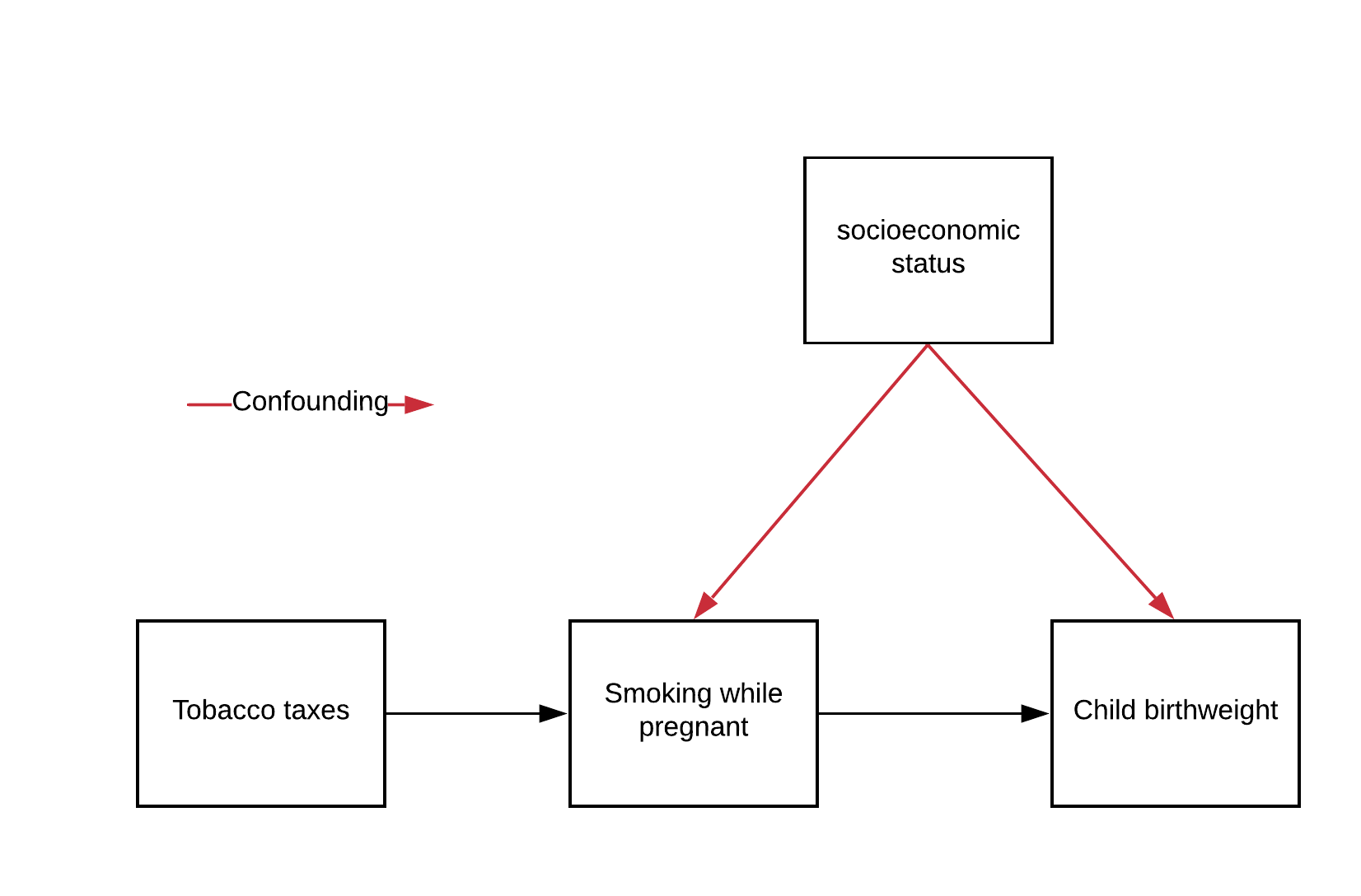
Section 1.1: A non-neuro example of an IV#
A classic example is estimating the effect of smoking cigarettes while pregnant on the birth weight of the infant. There is a (negative) correlation, but is it causal? Unfortunately many confounds affect both birth weight and smoking. Wealth is a big one.
Instead of controlling everything imaginable, one can find an IV. Here the instrumental variable is state taxes on tobacco. These
Are observable
Affect tobacco consumption
Don’t affect birth weight except through tobacco
By using the power of IV techniques, you can determine the causal effect without exhaustively controlling for everything.
Let’s represent our tobacco example above with the following notation:
\(Z_{\text{taxes}}\): our tobacco tax instrument, which only affects an individual’s tendency to smoke while pregnant within our system
\(T_{\text{smoking}}\): number of cigarettes smoked per day while pregnant, our “treatment” if this were a randomized trial
\(C_{\text{SES}}\): socioeconomic status (higher means wealthier), a confounder if it is not observed
\(Y_{\text{birthweight}}\): child birthweight in grams, our outcome of interest
Let’s suppose we have the following function for our system:
\(Y_{\text{birthweight}} = 3000 + C_{\text{SES}} - 2T_{\text{smoking}},\)
with the additional fact that \(C_{\text{SES}}\) is negatively correlated with \(T_{\text{smoking}}\).
The causal effect we wish to estimate is the coefficient \(-2\) for \(T_{\text{smoking}}\), which means that if a mother smokes one additional cigarette per day while pregnant her baby will be 2 grams lighter at birth.
We’ve provided a covariance matrix with the desired structure in the code cell below, so please run it to look at the correlations between our variables.
Execute this cell to see correlations with C
Show code cell source
# @markdown Execute this cell to see correlations with C
# run this code below to generate our setup
idx_dict = {
'Z': 0,
'T': 1,
'C': 2,
'Y': 3
}
# vars: Z T C
covar = np.array([[1.0, 0.5, 0.0], # Z
[0.5, 1.0, -0.5], # T
[0.0, -0.5, 1.0]]) # C
# vars: Z T C
means = [0, 5, 2]
# generate some data
np.random.seed(42)
data = np.random.multivariate_normal(mean=means, cov=2 * covar, size=2000)
# generate Y from our equation above
Y = 3000 + data[:, idx_dict['C']] - (2 * (data[:, idx_dict['T']]))
data = np.concatenate([data, Y.reshape(-1, 1)], axis=1)
Z = data[:, [idx_dict['Z']]]
T = data[:, [idx_dict['T']]]
C = data[:, [idx_dict['C']]]
Y = data[:, [idx_dict['Y']]]
corrs = np.corrcoef(data.transpose())
print_corr('C', 'T', corrs, idx_dict)
print_corr('C', 'Y', corrs, idx_dict)
Correlation between C and T (SES status and # cigarettes): -0.483
Correlation between C and Y (SES status and birth weight): 0.740
We see what is exactly represented in our graph above: \(C_{\text{SES}}\) is correlated with both \(T_{\text{smoking}}\) and \(Y_{\text{birthweight}}\), so \(C_{\text{SES}}\) is a potential confounder if not included in our analysis. Let’s say that it is difficult to observe and quantify \(C_{\text{SES}}\), so we do not have it available to regress against. This is another example of the omitted variable bias we saw in the last tutorial.
What about \(Z_{\text{taxes}}\)? Does it satisfy conditions 1, 2, and 3 of an instrument?
Execute this cell to see correlations of Z
Show code cell source
#@markdown Execute this cell to see correlations of Z
print("Condition 2?")
print_corr('Z', 'T', corrs, idx_dict)
print("Condition 3?")
print_corr('Z', 'C', corrs, idx_dict)
Condition 2?
Correlation between Z and T (taxes and # cigarettes): 0.519
Condition 3?
Correlation between Z and C (taxes and SES status): 0.009
Perfect! We see that \(Z_{\text{taxes}}\) is correlated with \(T_{\text{smoking}}\) (#2) but is uncorrelated with \(C_{\text{SES}}\) (#3). \(Z_\text{taxes}\) is also observable (#1), so we’ve satisfied our three criteria for an instrument:
\(Z_\text{taxes}\) is observable
\(Z_\text{taxes}\) affects \(T_{\text{smoking}}\)
\(Z_\text{taxes}\) doesn’t affect \(Y_{\text{birthweight}}\) except through \(T_{\text{smoking}}\) (ie \(Z_\text{taxes}\) doesn’t affect or is affected by \(C_\text{SES}\))
Section 1.2: How IV works, at high level#
The easiest way to imagine IV is that the instrument is an observable source of “randomness” that affects the treatment. In this way it’s similar to the interventions we talked about in Tutorial 1.
But how do you actually use the instrument? The key is that we need to extract the component of the treatment that is due only to the effect of the instrument. We will call this component \(\hat{T}\). $\( \hat{T}\leftarrow \text{The unconfounded component of }T \)\( Getting \)\hat{T}\( is fairly simple. It is simply the predicted value of \)T\( found in a regression that has only the instrument \)Z$ as input.
Once we have the unconfounded component in hand, getting the causal effect is as easy as regressing the outcome on \(\hat{T}\).
Section 1.3: IV estimation using two-stage least squares#
The fundamental technique for instrumental variable estimation is two-stage least squares.
We run two regressions:
The first stage gets \(\hat{T}_{\text{smoking}}\) by regressing \(T_{\text{smoking}}\) on \(Z_\text{taxes}\), fitting the parameter \(\hat{\alpha}\):
The second stage then regresses \(Y_{\text{birthweight}}\) on \(\hat{T}_{\text{smoking}}\) to obtain an estimate \(\hat{\beta}\) of the causal effect:
The first stage estimates the unconfounded component of \(T_{\text{smoking}}\) (ie, unaffected by the confounder \(C_{\text{SES}}\)), as we discussed above.
Then, the second stage uses this unconfounded component \(\hat{T}_{\text{smoking}}\) to estimate the effect of smoking on \(\hat{Y}_{\text{birthweight}}\).
We will explore how all this works in the next two exercises.
Section 1.3.1: Least squares regression stage 1#
Video 2: Stage 1#
Submit your feedback#
Show code cell source
# @title Submit your feedback
content_review(f"{feedback_prefix}_Stage_1_Video")
Coding Exercise 1.3.1: Compute regression stage 1#
Let’s run the regression of \(T_{\text{smoking}}\) on \(Z_\text{taxes}\) to compute \(\hat{T}_{\text{smoking}}\). We will then check whether our estimate is still confounded with \(C_{\text{SES}}\) by comparing the correlation of \(C_{\text{SES}}\) with \(T_{\text{smoking}}\) vs \(\hat{T}_{\text{smoking}}\).
Suggestions#
use the
LinearRegression()model, already imported from scikit-learnuse
fit_intercept=Trueas the only parameter settingbe sure to check the ordering of the parameters passed to
LinearRegression.fit()
def fit_first_stage(T, Z):
"""
Estimates T_hat as the first stage of a two-stage least squares.
Args:
T (np.ndarray): our observed, possibly confounded, treatment of shape (n, 1)
Z (np.ndarray): our observed instruments of shape (n, 1)
Returns
T_hat (np.ndarray): our estimate of the unconfounded portion of T
"""
############################################################################
## Insert your code here to fit the first stage of the 2-stage least squares
## estimate.
## Fill out function and remove
raise NotImplementedError('Please complete fit_first_stage function')
############################################################################
# Initialize linear regression model
stage1 = LinearRegression(...)
# Fit linear regression model
stage1.fit(...)
# Predict T_hat using linear regression model
T_hat = stage1.predict(...)
return T_hat
# Estimate T_hat
T_hat = fit_first_stage(T, Z)
# Get correlations
T_C_corr = np.corrcoef(T.transpose(), C.transpose())[0, 1]
T_hat_C_corr = np.corrcoef(T_hat.transpose(), C.transpose())[0, 1]
# Print correlations
print(f"Correlation between T and C: {T_C_corr:.3f}")
print(f"Correlation between T_hat and C: {T_hat_C_corr:.3f}")
You should see a correlation between \(T\) and \(C\) of -0.483 and between \(\hat{T}\) and \(C\) of 0.009.
Submit your feedback#
Show code cell source
# @title Submit your feedback
content_review(f"{feedback_prefix}_Compute_regression_stage_1_Exercise")
Section 1.3.2: Least squares regression stage 2#
Video 3: Stage 2#
Submit your feedback#
Show code cell source
# @title Submit your feedback
content_review(f"{feedback_prefix}_Stage_2_Video")
Coding Exercise 1.3.2: Compute the IV estimate#
Now let’s implement the second stage! Complete the fit_second_stage() function below. We will again use a linear regression model with an intercept. We will then use the function from Exercise 1 (fit_first_stage) and this function to estimate the full two-stage regression model. We will obtain the estimated causal effect of the number of cigarettes (\(T\)) on birth weight (\(Y\)).
def fit_second_stage(T_hat, Y):
"""
Estimates a scalar causal effect from 2-stage least squares regression using
an instrument.
Args:
T_hat (np.ndarray): the output of the first stage regression
Y (np.ndarray): our observed response (n, 1)
Returns:
beta (float): the estimated causal effect
"""
############################################################################
## Insert your code here to fit the second stage of the 2-stage least squares
## estimate.
## Fill out function and remove
raise NotImplementedError('Please complete fit_second_stage function')
############################################################################
# Initialize linear regression model
stage2 = LinearRegression(...)
# Fit model to data
stage2.fit(...)
return stage2.coef_
# Fit first stage
T_hat = fit_first_stage(T, Z)
# Fit second stage
beta = fit_second_stage(T_hat, Y)
# Print
print(f"Estimated causal effect is: {beta[0, 0]:.3f}")
You should obtain an estimated causal effect of -1.984. This is quite close to the true causal effect of \(-2\)!
Submit your feedback#
Show code cell source
# @title Submit your feedback
content_review(f"{feedback_prefix}_Compute_the_IV_estimate_Exercise")
Section 2: IVs in our simulated neural system#
Estimated timing to here from start of tutorial: 30 min
Video 4: IVs in simulated neural systems#
Submit your feedback#
Show code cell source
# @title Submit your feedback
content_review(f"{feedback_prefix}_ IVs_in_simulated_neural_systems_Video")
Now, say we have the neural system we have been simulating, except with an additional variable \(\vec{z}\). This will be our instrumental variable.
We treat \(\vec{z}\) as a source of noise in the dynamics of our neurons:
\(\eta\) is what we’ll call the “strength” of our IV
\(\vec{z}_t\) is a random binary variable, \(\vec{z}_t \sim Bernoulli(0.5)\)
Remember that for each neuron \(i\), we are trying to figure out whether \(i\) is connected to (causally affects) the other neurons in our system at the next time step. So for timestep \(t\), we want to determine whether \(\vec{x}_{i,t}\) affects all the other neurons at \(\vec{x}_{t+1}\). For a given neuron \(i\), \(\vec{z}_{i,t}\) satisfies the 3 criteria for a valid instrument.
What could \(z\) be, biologically?
Imagine \(z\) to be some injected current through an in vivo patch clamp. It affects each neuron individually, and only affects dynamics through that neuron.
The cool thing about IV is that you don’t have to control \(z\) yourself - it can be observed. So if you mess up your wiring and accidentally connect the injected voltage to an AM radio, no worries. As long as you can observe the signal the method will work.
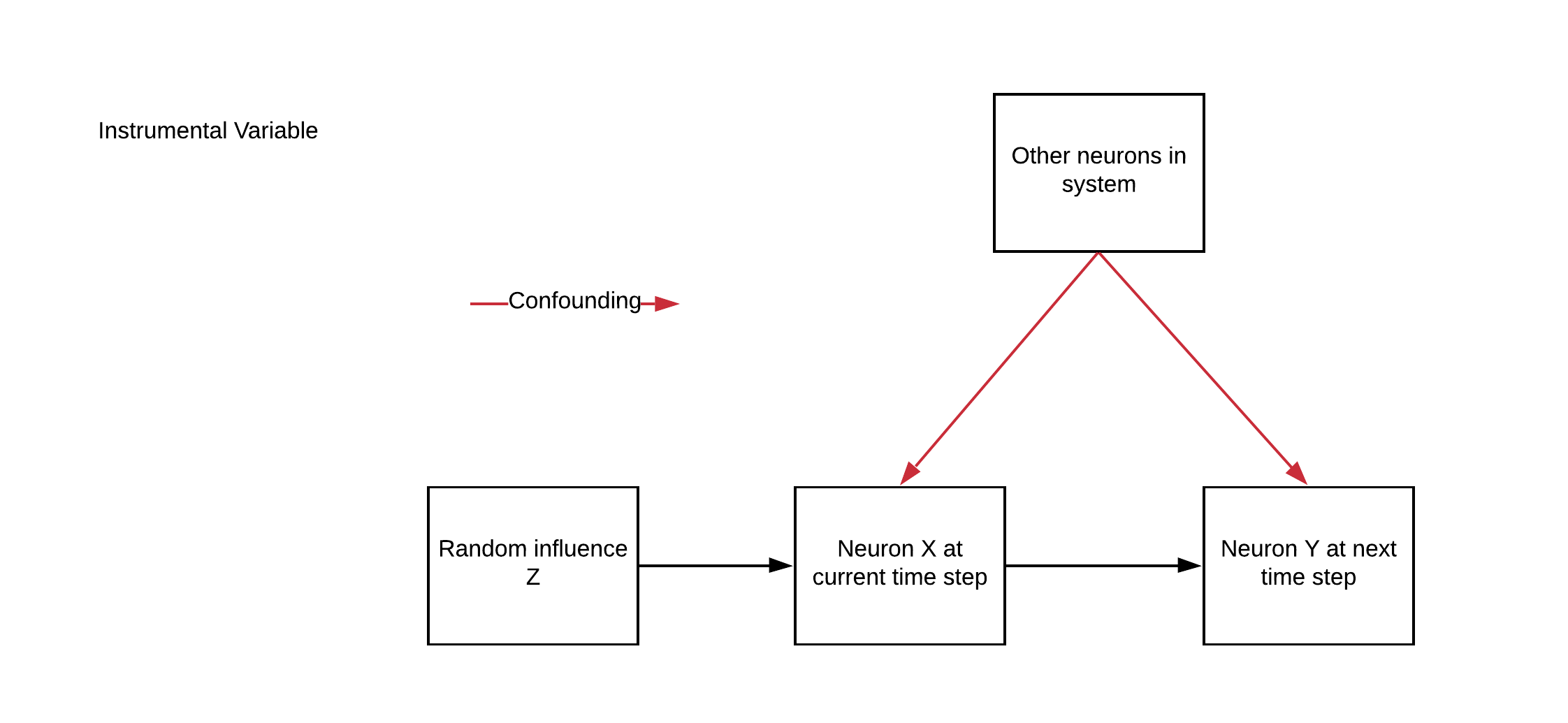
Section 2.1: Simulate a system with IV#
Coding Exercise 2.1: Simulate a system with IV#
Here we’ll modify the function that simulates the neural system, but this time make the update rule include the effect of the instrumental variable \(z\).
def simulate_neurons_iv(n_neurons, timesteps, eta, random_state=42):
"""
Simulates a dynamical system for the specified number of neurons and timesteps.
Args:
n_neurons (int): the number of neurons in our system.
timesteps (int): the number of timesteps to simulate our system.
eta (float): the strength of the instrument
random_state (int): seed for reproducibility
Returns:
The tuple (A,X,Z) of the connectivity matrix, simulated system, and instruments.
- A has shape (n_neurons, n_neurons)
- X has shape (n_neurons, timesteps)
- Z has shape (n_neurons, timesteps)
"""
np.random.seed(random_state)
A = create_connectivity(n_neurons, random_state)
X = np.zeros((n_neurons, timesteps))
Z = np.random.choice([0, 1], size=(n_neurons, timesteps))
for t in range(timesteps - 1):
############################################################################
## Insert your code here to adjust the update rule to include the
## instrumental variable.
## We've already created Z for you. (We need to return it to regress on it).
## Your task is to slice it appropriately. Don't forget eta.
## Fill out function and remove
raise NotImplementedError('Complete simulate_neurons_iv function')
############################################################################
IV_on_this_timestep = ...
X[:, t + 1] = sigmoid(A.dot(X[:, t]) + IV_on_this_timestep + np.random.multivariate_normal(np.zeros(n_neurons), np.eye(n_neurons)))
return A, X, Z
# Set parameters
timesteps = 5000 # Simulate for 5000 timesteps.
n_neurons = 100 # the size of our system
eta = 2 # the strength of our instrument, higher is stronger
# Simulate our dynamical system for the given amount of time
A, X, Z = simulate_neurons_iv(n_neurons, timesteps, eta)
# Visualize
plot_neural_activity(X)
Example output:
Submit your feedback#
Show code cell source
# @title Submit your feedback
content_review(f"{feedback_prefix}_Simulate_a_system_with_IV_Exercise")
Section 2.2: Estimate IV for simulated neural system#
Since you just implemented two-stage least squares, we’ve provided the network implementation for you, with the function get_iv_estimate_network(). Now, let’s see how our IV estimates do in recovering the connectivity matrix.
def get_iv_estimate_network(X, Z):
"""
Estimates the connectivity matrix from 2-stage least squares regression
using an instrument.
Args:
X (np.ndarray): our simulated system of shape (n_neurons, timesteps)
Z (np.ndarray): our observed instruments of shape (n_neurons, timesteps)
Returns:
V (np.ndarray): the estimated connectivity matrix
"""
n_neurons = X.shape[0]
Y = X[:, 1:].transpose()
# apply inverse sigmoid transformation
Y = logit(Y)
# Stage 1: regress X on Z
stage1 = MultiOutputRegressor(LinearRegression(fit_intercept=True), n_jobs=-1)
stage1.fit(Z[:, :-1].transpose(), X[:, :-1].transpose())
X_hat = stage1.predict(Z[:, :-1].transpose())
# Stage 2: regress Y on X_hatI
stage2 = MultiOutputRegressor(LinearRegression(fit_intercept=True), n_jobs=-1)
stage2.fit(X_hat, Y)
# Get estimated effects
V = np.zeros((n_neurons, n_neurons))
for i, estimator in enumerate(stage2.estimators_):
V[i, :] = estimator.coef_
return V
Now let’s see how well it works in our system.
Execute this cell to visualize IV estimated connectivity matrix
Show code cell source
# @markdown Execute this cell to visualize IV estimated connectivity matrix
n_neurons = 6
timesteps = 10000
random_state = 42
eta = 2
A, X, Z = simulate_neurons_iv(n_neurons, timesteps, eta, random_state)
V = get_iv_estimate_network(X, Z)
corr_ = np.corrcoef(A.flatten(), V.flatten())[1, 0]
fig, axs = plt.subplots(1, 2, figsize=(10, 5))
im = axs[0].imshow(A, cmap="coolwarm")
fig.colorbar(im, ax=axs[0],fraction=0.046, pad=0.04)
axs[0].title.set_text("True connectivity matrix")
axs[0].set(xlabel='Connectivity from', ylabel='Connectivity to')
im = axs[1].imshow(V, cmap="coolwarm")
fig.colorbar(im, ax=axs[1],fraction=0.046, pad=0.04)
axs[1].title.set_text("IV estimated connectivity matrix")
axs[1].set(xlabel='Connectivity from')
fig.suptitle(f"IV estimated correlation: {corr_:.3f}")
plt.show()
The IV estimates seem to perform pretty well! In the next section, we will see how they behave in the face of omitted variable bias.
Section 3: IVs and omitted variable bias#
Estimated timing to here from start of tutorial: 40 min
Video 5: IV vs regression#
Submit your feedback#
Show code cell source
# @title Submit your feedback
content_review(f"{feedback_prefix}_IVs_and_omitted_variable_bias_Video")
Interactive Demo 3: Estimating connectivity with IV vs regression on a subset of observed neurons#
Change the ratio of observed neurons and look at the impact on the quality of connectivity estimation using IV vs regression. Which method does better with fewer observed neurons?
Show code cell source
# @markdown Execute this cell to enable the widget.
# @markdown This simulation will take about a minute to run!
n_neurons = 30
timesteps = 20000
random_state = 42
eta = 2
A, X, Z = simulate_neurons_iv(n_neurons, timesteps, eta, random_state)
reg_args = {
"fit_intercept": False,
"alpha": 0.001
}
@widgets.interact
def plot_observed(ratio=[0.2, 0.4, 0.6, 0.8, 1.0]):
fig, axs = plt.subplots(1, 3, figsize=(15, 5))
sel_idx = int(ratio * n_neurons)
n_observed = sel_idx
offset = np.zeros((n_neurons, n_neurons))
offset[:sel_idx, :sel_idx] = 1 + A[:sel_idx, :sel_idx]
im = axs[0].imshow(offset, cmap="coolwarm", vmin=0, vmax=A.max() + 1)
axs[0].title.set_text("True connectivity")
axs[0].set_xlabel("Connectivity to")
axs[0].set_ylabel("Connectivity from")
plt.colorbar(im, ax=axs[0],fraction=0.046, pad=0.04)
sel_A = A[:sel_idx, :sel_idx]
sel_X = X[:sel_idx, :]
sel_Z = Z[:sel_idx, :]
V = get_iv_estimate_network(sel_X, sel_Z)
iv_corr = np.corrcoef(sel_A.flatten(), V.flatten())[1, 0]
big_V = np.zeros(A.shape)
big_V[:sel_idx, :sel_idx] = 1 + V
im = axs[1].imshow(big_V, cmap="coolwarm", vmin=0, vmax=A.max() + 1)
plt.colorbar(im, ax=axs[1], fraction=0.046, pad=0.04)
c = 'w' if n_observed < (n_neurons - 3) else 'k'
axs[1].text(0, n_observed + 2, f"Correlation: {iv_corr:.2f}",
color=c, size=15)
axs[1].axis("off")
reg_corr, R = get_regression_corr_full_connectivity(n_neurons, A, X, ratio,
reg_args)
big_R = np.zeros(A.shape)
big_R[:sel_idx, :sel_idx] = 1 + R
im = axs[2].imshow(big_R, cmap="coolwarm", vmin=0, vmax=A.max() + 1)
plt.colorbar(im, ax=axs[2], fraction=0.046, pad=0.04)
c = 'w' if n_observed<(n_neurons-3) else 'k'
axs[1].title.set_text("Estimated connectivity (IV)")
axs[1].set_xlabel("Connectivity to")
axs[1].set_ylabel("Connectivity from")
axs[2].text(0, n_observed + 2, f"Correlation: {reg_corr:.2f}",
color=c, size=15)
axs[2].axis("off")
axs[2].title.set_text("Estimated connectivity (regression)")
axs[2].set_xlabel("Connectivity to")
axs[2].set_ylabel("Connectivity from")
plt.show()
We can also visualize the performance of regression and IV as a function of the observed neuron ratio below.
Note that this code takes about a minute to run!
Execute this cell to visualize connectivity estimation performance
Show code cell source
# @markdown Execute this cell to visualize connectivity estimation performance
def compare_iv_estimate_to_regression(observed_ratio):
"""
A wrapper function to compare IV and Regressor performance as a function of
observed neurons
Args:
observed_ratio(list): a list of different observed ratios
(out of the whole system)
"""
# Let's compare IV estimates to our regression estimates
reg_corrs = np.zeros((len(observed_ratio),))
iv_corrs = np.zeros((len(observed_ratio),))
for j, ratio in enumerate(observed_ratio):
sel_idx = int(ratio * n_neurons)
sel_X = X[:sel_idx, :]
sel_Z = X[:sel_idx, :]
sel_A = A[:sel_idx, :sel_idx]
sel_reg_V = get_regression_estimate(sel_X)
reg_corrs[j] = np.corrcoef(sel_A.flatten(), sel_reg_V.flatten())[1, 0]
sel_iv_V = get_iv_estimate_network(sel_X, sel_Z)
iv_corrs[j] = np.corrcoef(sel_A.flatten(), sel_iv_V.flatten())[1, 0]
# Plotting IV vs lasso performance
plt.plot(observed_ratio, reg_corrs)
plt.plot(observed_ratio, iv_corrs)
plt.xlim([1, 0.2])
plt.ylabel("Connectivity matrices\ncorrelation with truth")
plt.xlabel("Fraction of observed variables")
plt.title("IV and lasso performance as a function of observed neuron ratio")
plt.legend(['Regression', 'IV'])
plt.show()
n_neurons = 40 # the size of the system
timesteps = 20000
random_state = 42
eta = 2 # the strength of our instrument
A, X, Z = simulate_neurons_iv(n_neurons, timesteps, eta, random_state)
observed_ratio = [1, 0.8, 0.6, 0.4, 0.2]
compare_iv_estimate_to_regression(observed_ratio)
We see that IVs handle omitted variable bias (when the instrument is strong and we have enough data).
The costs of IV analysis
we need to find an appropriate and valid instrument
Because of the 2-stage estimation process, we need strong instruments or else our standard errors will be large
Submit your feedback#
Show code cell source
# @title Submit your feedback
content_review(f"{feedback_prefix}_Estimating_connectivity_with_IV_vs_regression_Interactive_Demo")
Section 4: Thinking about causality in your work#
Estimated timing to here from start of tutorial: 50 min
Think 4!: Discussion questions#
Please discuss the following in groups for around 10 minutes.
Think back to your most recent work. Can you create a causal diagram of the fundamental question? Are there sources of bias (omitted variables or otherwise) that might be a threat to causal validity?
Can you think of any possibilities for instrumental variables? What sources of observed randomness could studies in your field leverage in identifying causal effects?
Submit your feedback#
Show code cell source
# @title Submit your feedback
content_review(f"{feedback_prefix}_ Discussion_questions_Discussion")
Summary#
Estimated timing of tutorial: 1 hour, 5 min
Video 6: Summary#
Submit your feedback#
Show code cell source
# @title Submit your feedback
content_review(f"{feedback_prefix}_Summary_Video")
In this tutorial, we:
Explored instrumental variables and how we can use them for causality estimates
Compared IV estimates to regression estimates
Bonus#
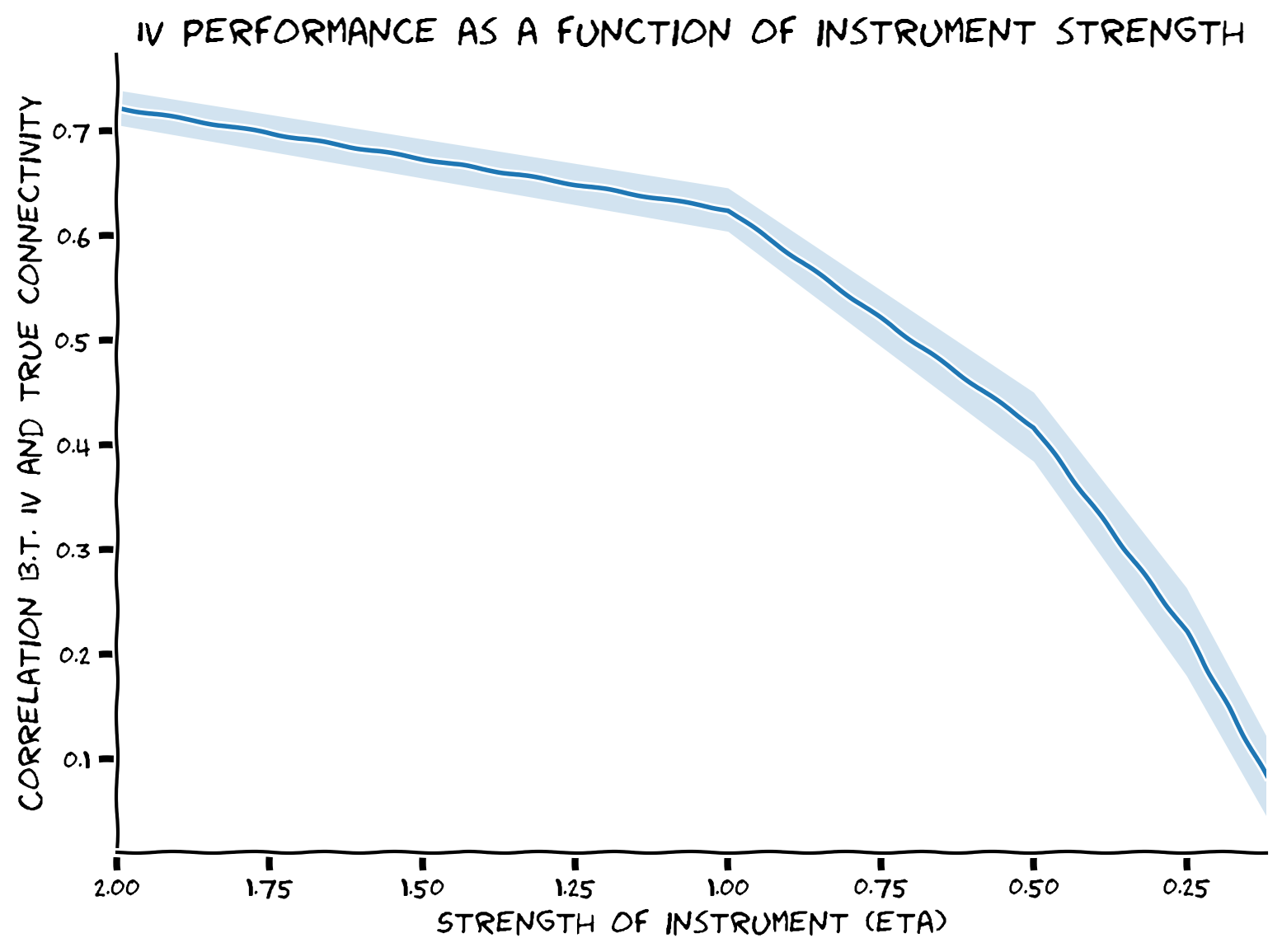
Bonus Section 1: Exploring Instrument Strength#
Bonus Coding Exercise 1: Exploring instrument strength#
Explore how the strength of the instrument \(\eta\) affects the quality of estimates with instrumental variables.
def instrument_strength_effect(etas, n_neurons, timesteps, n_trials):
""" Compute IV estimation performance for different instrument strengths
Args:
etas (list): different instrument strengths to compare
n_neurons (int): number of neurons in simulation
timesteps (int): number of timesteps in simulation
n_trials (int): number of trials to compute
Returns:
ndarray: n_trials x len(etas) array where each element is the correlation
between true and estimated connectivity matrices for that trial and
instrument strength
"""
# Initialize corr array
corr_data = np.zeros((n_trials, len(etas)))
# Loop over trials
for trial in range(n_trials):
print(f"simulation of trial {trial + 1} of {n_trials}")
# Loop over instrument strengths
for j, eta in enumerate(etas):
########################################################################
## TODO: Simulate system with a given instrument strength, get IV estimate,
## and compute correlation
# Fill out function and remove
raise NotImplementedError('Student exercise: complete instrument_strength_effect')
########################################################################
# Simulate system
A, X, Z = simulate_neurons_iv(...)
# Compute IV estimate
iv_V = get_iv_estimate_network(...)
# Compute correlation
corr_data[trial, j] = np.corrcoef(A.flatten(), iv_V.flatten())[1, 0]
return corr_data
# Parameters of system
n_neurons = 20
timesteps = 10000
n_trials = 3
etas = [2, 1, 0.5, 0.25, 0.12] # instrument strengths to search over
# Get IV estimate performances
corr_data = instrument_strength_effect(etas, n_neurons, timesteps, n_trials)
# Visualize
plot_performance_vs_eta(etas, corr_data)
Example output:
Submit your feedback#
Show code cell source
# @title Submit your feedback
content_review(f"{feedback_prefix}_Exploring_instrument_strength_Bonus_Exercise")
Bonus Section 2: Granger Causality#
Another potential solution to temporal causation that we might consider: Granger Causality.
But, like the simultaneous fitting we explored in Tutorial 3, this method still fails in the presence of unobserved variables.
We are testing whether a time series \(X\) Granger-causes a time series \(Y\) through a hypothesis test:
the null hypothesis \(H_0\): lagged values of \(X\) do not help predict values of \(Y\)
the alternative hypothesis \(H_a\): lagged values of \(X\) do help predict values of \(Y\)
Mechanically, this is accomplished by fitting autoregressive models for \(y_{t}\). We fail to reject the hypothesis if none of the \(x_{t-k}\) terms are retained as significant in the regression. For simplicity, we will consider only one time lag. So, we have:
Execute this cell to get custom imports from statsmodels library
Show code cell source
# @markdown Execute this cell to get custom imports from [statsmodels](https://www.statsmodels.org/stable/index.html) library
!pip install statsmodels --quiet
from statsmodels.tsa.stattools import grangercausalitytests
Bonus Section 2.1: Granger causality in small systems#
We will first evaluate Granger causality in a small system.
Bonus Coding Exercise 2.1: Evaluate Granger causality#
Complete the following definition to evaluate the Granger causality between our neurons. Then run the cells below to evaluate how well it works. You will use the grangercausalitytests() function already imported from statsmodels. We will then check whether a neuron in a small system Granger-causes the others.
def get_granger_causality(X, selected_neuron, alpha=0.05):
"""
Estimates the lag-1 granger causality of the given neuron on the other neurons in the system.
Args:
X (np.ndarray): the matrix holding our dynamical system of shape (n_neurons, timesteps)
selected_neuron (int): the index of the neuron we want to estimate granger causality for
alpha (float): Bonferroni multiple comparisons correction
Returns:
A tuple (reject_null, p_vals)
reject_null (list): a binary list of length n_neurons whether the null was
rejected for the selected neuron granger causing the other neurons
p_vals (list): a list of the p-values for the corresponding Granger causality tests
"""
n_neurons = X.shape[0]
max_lag = 1
reject_null = []
p_vals = []
for target_neuron in range(n_neurons):
ts_data = X[[target_neuron, selected_neuron], :].transpose()
########################################################################
## Insert your code here to run Granger causality tests.
##
## Function Hints:
## Pass the ts_data defined above as the first argument
## Granger causality -> grangercausalitytests
## Fill out this function and then remove
raise NotImplementedError('Student exercise: complete get_granger_causality function')
########################################################################
res = grangercausalitytests(...)
# Gets the p-value for the log-ratio test
pval = res[1][0]['lrtest'][1]
p_vals.append(pval)
reject_null.append(int(pval < alpha))
return reject_null, p_vals
# Set up small system
n_neurons = 6
timesteps = 5000
random_state = 42
selected_neuron = 1
A = create_connectivity(n_neurons, random_state)
X = simulate_neurons(A, timesteps, random_state)
# Estimate Granger causality
reject_null, p_vals = get_granger_causality(X, selected_neuron)
# Visualize
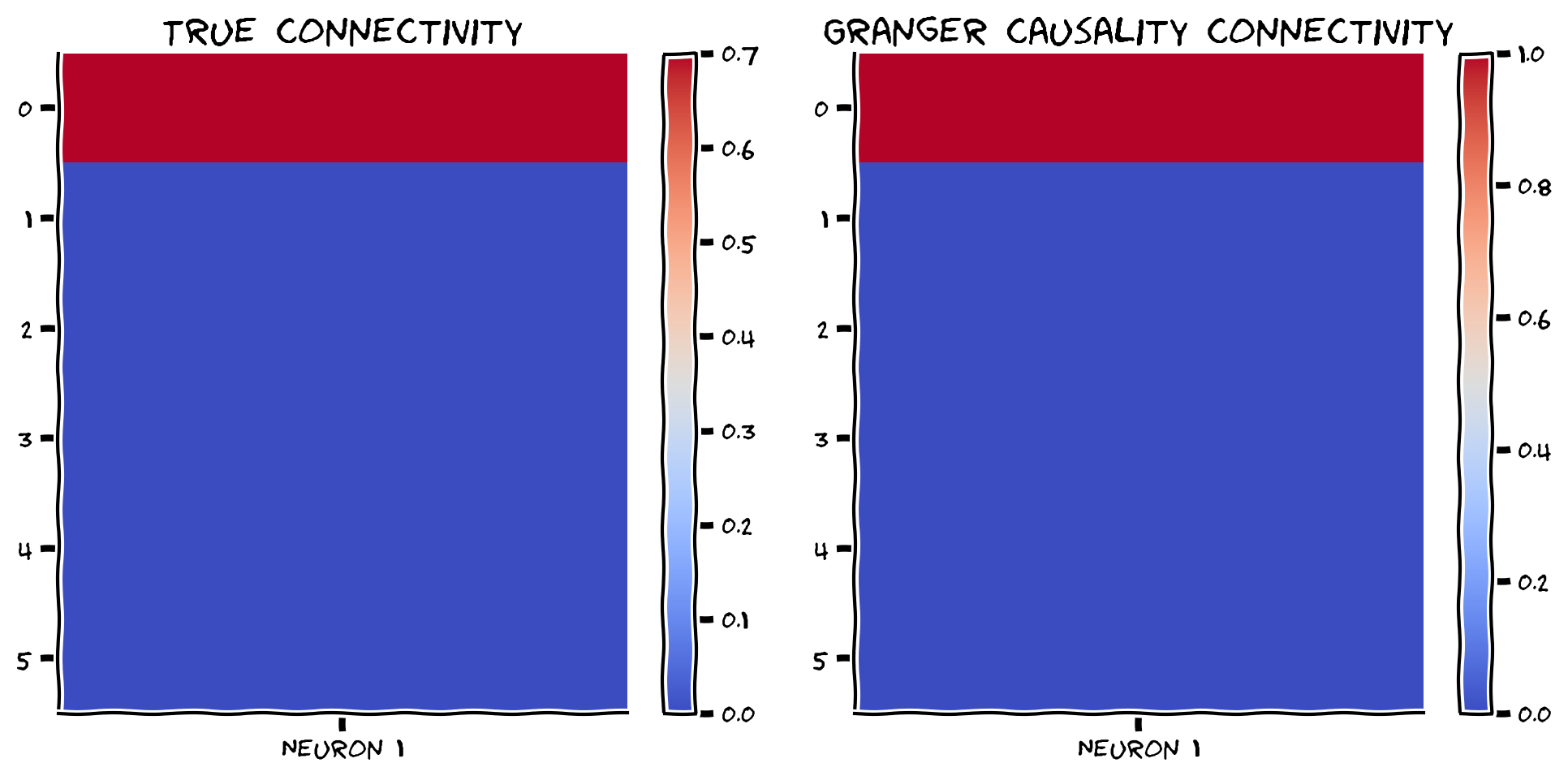
compare_granger_connectivity(A, reject_null, selected_neuron)
Example output:
Submit your feedback#
Show code cell source
# @title Submit your feedback
content_review(f"{feedback_prefix}_Evaluate_Granger_causality_Bonus_Exercise")
Looks good! Let’s also check the correlation between Granger estimates and the true connectivity.
print(np.corrcoef(A[:, selected_neuron], np.array(reject_null))[1, 0])
When we have a small system, we correctly identify the causality of neuron 1.
Bonus Section 2.2: Granger causality in large systems#
We will now run Granger causality on a large system with 100 neurons. Does it still work well? How does the number of timesteps matter?
Execute this cell to examine Granger causality in a large system
Show code cell source
# @markdown Execute this cell to examine Granger causality in a large system
n_neurons = 100
timesteps = 5000
random_state = 42
selected_neuron = 1
A = create_connectivity(n_neurons, random_state)
X = simulate_neurons(A, timesteps, random_state)
# get granger causality estimates
reject_null, p_vals = get_granger_causality(X, selected_neuron)
compare_granger_connectivity(A, reject_null, selected_neuron)
Let’s again check the correlation between the Granger estimates and the true connectivity. Are we able to recover the true connectivity well in this larger system?
print(np.corrcoef(A[:, selected_neuron], np.array(reject_null))[1, 0])
Notes on Granger Causality
Here we considered bivariate Granger causality – for each pair of neurons \(A, B\), does one Granger-cause the other? You might wonder whether considering more variables will help with estimation. Conditional Granger Causality is a technique that allows for a multivariate system, where we test whether \(A\) Granger-causes \(B\) conditional on the other variables in the system.
Even after controlling for variables in the system, conditional Granger causality will also likely perform poorly as our system gets larger. Plus, measuring the additional variables to condition on may be infeasible in practical applications, which would introduce omitted variable bias as we saw in the regression exercise.
One takeaway here is that as our estimation procedures become more sophisticated, they also become more difficult to interpret. We always need to understand the methods and the assumptions that are made.

