Bonus Tutorial: Extending the Wilson-Cowan Model#
Week 2, Day 4: Dynamic Networks
By Neuromatch Academy
Content creators: Qinglong Gu, Songtin Li, Arvind Kumar, John Murray, Julijana Gjorgjieva
Content reviewers: Maryam Vaziri-Pashkam, Ella Batty, Lorenzo Fontolan, Richard Gao, Spiros Chavlis, Michael Waskom
Production editors: Siddharth Suresh, Gagana B, Spiros Chavlis
Tutorial Objectives#
In the previous tutorial, you learned about the Wilson-Cowan rate model. Here we will dive into some deeper analyses of this model.
Bonus steps:
Find and plot the fixed points of the Wilson-Cowan model.
Investigate the stability of the Wilson-Cowan model by linearizing its dynamics and examining the Jacobian matrix.
Learn how the Wilson-Cowan model can reach an oscillatory state.
Applications of Wilson-Cowan model:
Visualize the behavior of an Inhibition-stabilized network.
Simulate working memory using the Wilson-Cowan model.
Reference paper:
Wilson, H., and Cowan, J. (1972). Excitatory and inhibitory interactions in localized populations of model neurons. Biophysical Journal 12. doi: 10.1016/S0006-3495(72)86068-5
Setup#
Install and import feedback gadget#
Show code cell source
# @title Install and import feedback gadget
!pip3 install vibecheck datatops --quiet
from vibecheck import DatatopsContentReviewContainer
def content_review(notebook_section: str):
return DatatopsContentReviewContainer(
"", # No text prompt
notebook_section,
{
"url": "https://pmyvdlilci.execute-api.us-east-1.amazonaws.com/klab",
"name": "neuromatch_cn",
"user_key": "y1x3mpx5",
},
).render()
feedback_prefix = "W2D4_T3_Bonus"
# Imports
import matplotlib.pyplot as plt
import numpy as np
import scipy.optimize as opt # root-finding algorithm
Figure Settings#
Show code cell source
# @title Figure Settings
import logging
logging.getLogger('matplotlib.font_manager').disabled = True
import ipywidgets as widgets # interactive display
%config InlineBackend.figure_format = 'retina'
plt.style.use("https://raw.githubusercontent.com/NeuromatchAcademy/course-content/main/nma.mplstyle")
Plotting Functions#
Show code cell source
# @title Plotting Functions
def plot_FI_inverse(x, a, theta):
f, ax = plt.subplots()
ax.plot(x, F_inv(x, a=a, theta=theta))
ax.set(xlabel="$x$", ylabel="$F^{-1}(x)$")
def plot_FI_EI(x, FI_exc, FI_inh):
plt.figure()
plt.plot(x, FI_exc, 'b', label='E population')
plt.plot(x, FI_inh, 'r', label='I population')
plt.legend(loc='lower right')
plt.xlabel('x (a.u.)')
plt.ylabel('F(x)')
plt.show()
def my_test_plot(t, rE1, rI1, rE2, rI2):
plt.figure()
ax1 = plt.subplot(211)
ax1.plot(pars['range_t'], rE1, 'b', label='E population')
ax1.plot(pars['range_t'], rI1, 'r', label='I population')
ax1.set_ylabel('Activity')
ax1.legend(loc='best')
ax2 = plt.subplot(212, sharex=ax1, sharey=ax1)
ax2.plot(pars['range_t'], rE2, 'b', label='E population')
ax2.plot(pars['range_t'], rI2, 'r', label='I population')
ax2.set_xlabel('t (ms)')
ax2.set_ylabel('Activity')
ax2.legend(loc='best')
plt.tight_layout()
plt.show()
def plot_nullclines(Exc_null_rE, Exc_null_rI, Inh_null_rE, Inh_null_rI):
plt.figure()
plt.plot(Exc_null_rE, Exc_null_rI, 'b', label='E nullcline')
plt.plot(Inh_null_rE, Inh_null_rI, 'r', label='I nullcline')
plt.xlabel(r'$r_E$')
plt.ylabel(r'$r_I$')
plt.legend(loc='best')
plt.show()
def my_plot_nullcline(pars):
Exc_null_rE = np.linspace(-0.01, 0.96, 100)
Exc_null_rI = get_E_nullcline(Exc_null_rE, **pars)
Inh_null_rI = np.linspace(-.01, 0.8, 100)
Inh_null_rE = get_I_nullcline(Inh_null_rI, **pars)
plt.plot(Exc_null_rE, Exc_null_rI, 'b', label='E nullcline')
plt.plot(Inh_null_rE, Inh_null_rI, 'r', label='I nullcline')
plt.xlabel(r'$r_E$')
plt.ylabel(r'$r_I$')
plt.legend(loc='best')
def my_plot_vector(pars, my_n_skip=2, myscale=5):
EI_grid = np.linspace(0., 1., 20)
rE, rI = np.meshgrid(EI_grid, EI_grid)
drEdt, drIdt = EIderivs(rE, rI, **pars)
n_skip = my_n_skip
plt.quiver(rE[::n_skip, ::n_skip], rI[::n_skip, ::n_skip],
drEdt[::n_skip, ::n_skip], drIdt[::n_skip, ::n_skip],
angles='xy', scale_units='xy', scale=myscale, facecolor='c')
plt.xlabel(r'$r_E$')
plt.ylabel(r'$r_I$')
def my_plot_trajectory(pars, mycolor, x_init, mylabel):
pars = pars.copy()
pars['rE_init'], pars['rI_init'] = x_init[0], x_init[1]
rE_tj, rI_tj = simulate_wc(**pars)
plt.plot(rE_tj, rI_tj, color=mycolor, label=mylabel)
plt.plot(x_init[0], x_init[1], 'o', color=mycolor, ms=8)
plt.xlabel(r'$r_E$')
plt.ylabel(r'$r_I$')
def my_plot_trajectories(pars, dx, n, mylabel):
"""
Solve for I along the E_grid from dE/dt = 0.
Expects:
pars : Parameter dictionary
dx : increment of initial values
n : n*n trjectories
mylabel : label for legend
Returns:
figure of trajectory
"""
pars = pars.copy()
for ie in range(n):
for ii in range(n):
pars['rE_init'], pars['rI_init'] = dx * ie, dx * ii
rE_tj, rI_tj = simulate_wc(**pars)
if (ie == n-1) & (ii == n-1):
plt.plot(rE_tj, rI_tj, 'gray', alpha=0.8, label=mylabel)
else:
plt.plot(rE_tj, rI_tj, 'gray', alpha=0.8)
plt.xlabel(r'$r_E$')
plt.ylabel(r'$r_I$')
def plot_complete_analysis(pars):
plt.figure(figsize=(7.7, 6.))
# plot example trajectories
my_plot_trajectories(pars, 0.2, 6,
'Sample trajectories \nfor different init. conditions')
my_plot_trajectory(pars, 'orange', [0.6, 0.8],
'Sample trajectory for \nlow activity')
my_plot_trajectory(pars, 'm', [0.6, 0.6],
'Sample trajectory for \nhigh activity')
# plot nullclines
my_plot_nullcline(pars)
# plot vector field
EI_grid = np.linspace(0., 1., 20)
rE, rI = np.meshgrid(EI_grid, EI_grid)
drEdt, drIdt = EIderivs(rE, rI, **pars)
n_skip = 2
plt.quiver(rE[::n_skip, ::n_skip], rI[::n_skip, ::n_skip],
drEdt[::n_skip, ::n_skip], drIdt[::n_skip, ::n_skip],
angles='xy', scale_units='xy', scale=5., facecolor='c')
plt.legend(loc=[1.02, 0.57], handlelength=1)
plt.show()
def plot_fp(x_fp, position=(0.02, 0.1), rotation=0):
plt.plot(x_fp[0], x_fp[1], 'ko', ms=8)
plt.text(x_fp[0] + position[0], x_fp[1] + position[1],
f'Fixed Point1=\n({x_fp[0]:.3f}, {x_fp[1]:.3f})',
horizontalalignment='center', verticalalignment='bottom',
rotation=rotation)
Helper functions#
Show code cell source
# @title Helper functions
def default_pars(**kwargs):
pars = {}
# Excitatory parameters
pars['tau_E'] = 1. # Timescale of the E population [ms]
pars['a_E'] = 1.2 # Gain of the E population
pars['theta_E'] = 2.8 # Threshold of the E population
# Inhibitory parameters
pars['tau_I'] = 2.0 # Timescale of the I population [ms]
pars['a_I'] = 1.0 # Gain of the I population
pars['theta_I'] = 4.0 # Threshold of the I population
# Connection strength
pars['wEE'] = 9. # E to E
pars['wEI'] = 4. # I to E
pars['wIE'] = 13. # E to I
pars['wII'] = 11. # I to I
# External input
pars['I_ext_E'] = 0.
pars['I_ext_I'] = 0.
# simulation parameters
pars['T'] = 50. # Total duration of simulation [ms]
pars['dt'] = .1 # Simulation time step [ms]
pars['rE_init'] = 0.2 # Initial value of E
pars['rI_init'] = 0.2 # Initial value of I
# External parameters if any
for k in kwargs:
pars[k] = kwargs[k]
# Vector of discretized time points [ms]
pars['range_t'] = np.arange(0, pars['T'], pars['dt'])
return pars
def F(x, a, theta):
"""
Population activation function, F-I curve
Args:
x : the population input
a : the gain of the function
theta : the threshold of the function
Returns:
f : the population activation response f(x) for input x
"""
# add the expression of f = F(x)
f = (1 + np.exp(-a * (x - theta)))**-1 - (1 + np.exp(a * theta))**-1
return f
def dF(x, a, theta):
"""
Derivative of the population activation function.
Args:
x : the population input
a : the gain of the function
theta : the threshold of the function
Returns:
dFdx : Derivative of the population activation function.
"""
dFdx = a * np.exp(-a * (x - theta)) * (1 + np.exp(-a * (x - theta)))**-2
return dFdx
def F_inv(x, a, theta):
"""
Args:
x : the population input
a : the gain of the function
theta : the threshold of the function
Returns:
F_inverse : value of the inverse function
"""
# Calculate Finverse (ln(x) can be calculated as np.log(x))
F_inverse = -1/a * np.log((x + (1 + np.exp(a * theta))**-1)**-1 - 1) + theta
return F_inverse
def get_E_nullcline(rE, a_E, theta_E, wEE, wEI, I_ext_E, **other_pars):
"""
Solve for rI along the rE from drE/dt = 0.
Args:
rE : response of excitatory population
a_E, theta_E, wEE, wEI, I_ext_E : Wilson-Cowan excitatory parameters
Other parameters are ignored
Returns:
rI : values of inhibitory population along the nullcline on the rE
"""
# calculate rI for E nullclines on rI
rI = 1 / wEI * (wEE * rE - F_inv(rE, a_E, theta_E) + I_ext_E)
return rI
def get_I_nullcline(rI, a_I, theta_I, wIE, wII, I_ext_I, **other_pars):
"""
Solve for E along the rI from dI/dt = 0.
Args:
rI : response of inhibitory population
a_I, theta_I, wIE, wII, I_ext_I : Wilson-Cowan inhibitory parameters
Other parameters are ignored
Returns:
rE : values of the excitatory population along the nullcline on the rI
"""
# calculate rE for I nullclines on rI
rE = 1 / wIE * (wII * rI + F_inv(rI, a_I, theta_I) - I_ext_I)
return rE
def EIderivs(rE, rI,
tau_E, a_E, theta_E, wEE, wEI, I_ext_E,
tau_I, a_I, theta_I, wIE, wII, I_ext_I,
**other_pars):
"""Time derivatives for E/I variables (dE/dt, dI/dt)."""
# Compute the derivative of rE
drEdt = (-rE + F(wEE * rE - wEI * rI + I_ext_E, a_E, theta_E)) / tau_E
# Compute the derivative of rI
drIdt = (-rI + F(wIE * rE - wII * rI + I_ext_I, a_I, theta_I)) / tau_I
return drEdt, drIdt
def simulate_wc(tau_E, a_E, theta_E, tau_I, a_I, theta_I,
wEE, wEI, wIE, wII, I_ext_E, I_ext_I,
rE_init, rI_init, dt, range_t, **other_pars):
"""
Simulate the Wilson-Cowan equations
Args:
Parameters of the Wilson-Cowan model
Returns:
rE, rI (arrays) : Activity of excitatory and inhibitory populations
"""
# Initialize activity arrays
Lt = range_t.size
rE = np.append(rE_init, np.zeros(Lt - 1))
rI = np.append(rI_init, np.zeros(Lt - 1))
I_ext_E = I_ext_E * np.ones(Lt)
I_ext_I = I_ext_I * np.ones(Lt)
# Simulate the Wilson-Cowan equations
for k in range(Lt - 1):
# Calculate the derivative of the E population
drE = dt / tau_E * (-rE[k] + F(wEE * rE[k] - wEI * rI[k] + I_ext_E[k],
a_E, theta_E))
# Calculate the derivative of the I population
drI = dt / tau_I * (-rI[k] + F(wIE * rE[k] - wII * rI[k] + I_ext_I[k],
a_I, theta_I))
# Update using Euler's method
rE[k + 1] = rE[k] + drE
rI[k + 1] = rI[k] + drI
return rE, rI
The helper functions included:
Parameter dictionary:
default_pars(**kwargs). You can use:pars = default_pars()to get all the parameters, and then you can executeprint(pars)to check these parameters.pars = default_pars(T=T_sim, dt=time_step)to set a different simulation time and time stepAfter
pars = default_pars(), usepar['New_para'] = valueto add a new parameter with its valuePass to functions that accept individual parameters with
func(**pars)
F-I curve:
F(x, a, theta)Derivative of the F-I curve:
dF(x, a, theta)Inverse of F-I curve:
F_invNullcline calculations:
get_E_nullcline,get_I_nullclineDerivatives of E/I variables:
EIderivsSimulate the Wilson-Cowan model:
simulate_wc
Section 1: Fixed points, stability analysis, and limit cycles in the Wilson-Cowan model#
Correction to video: this is now the first part of the second bonus tutorial, not the last part of the second tutorial
Video 1: Fixed points and their stability#
Submit your feedback#
Show code cell source
# @title Submit your feedback
content_review(f"{feedback_prefix}_Fixed_points_and_stability_Video")
As in Tutorial 2, we will be looking at the Wilson-Cowan model, with coupled equations representing the dynamics of the excitatory or inhibitory population:
\(r_E(t)\) represents the average activation (or firing rate) of the excitatory population at time \(t\), and \(r_I(t)\) the activation (or firing rate) of the inhibitory population. The parameters \(\tau_E\) and \(\tau_I\) control the timescales of the dynamics of each population. Connection strengths are given by: \(w_{EE}\) (E \(\rightarrow\) E), \(w_{EI}\) (I \(\rightarrow\) E), \(w_{IE}\) (E \(\rightarrow\) I), and \(w_{II}\) (I \(\rightarrow\) I). The terms \(w_{EI}\) and \(w_{IE}\) represent connections from inhibitory to excitatory population and vice versa, respectively. The transfer functions (or F-I curves) \(F_E(x;a_E,\theta_E)\) and \(F_I(x;a_I,\theta_I)\) can be different for the excitatory and the inhibitory populations.
Section 1.1: Fixed Points of the E/I system#
The intersection points of the two nullcline curves are the fixed points of the Wilson-Cowan model in Equation \((1)\).
In the next exercise, we will find the coordinate of all fixed points for a given set of parameters.
We’ll make use of two functions, similar to ones we saw in Tutorial 1, which use a root-finding algorithm to find the fixed points of the system with Excitatory and Inhibitory populations.
Execute to visualize nullclines
Show code cell source
# @markdown Execute to visualize nullclines
# Set parameters
pars = default_pars()
Exc_null_rE = np.linspace(-0.01, 0.96, 100)
Inh_null_rI = np.linspace(-.01, 0.8, 100)
# Compute nullclines
Exc_null_rI = get_E_nullcline(Exc_null_rE, **pars)
Inh_null_rE = get_I_nullcline(Inh_null_rI, **pars)
plot_nullclines(Exc_null_rE, Exc_null_rI, Inh_null_rE, Inh_null_rI)
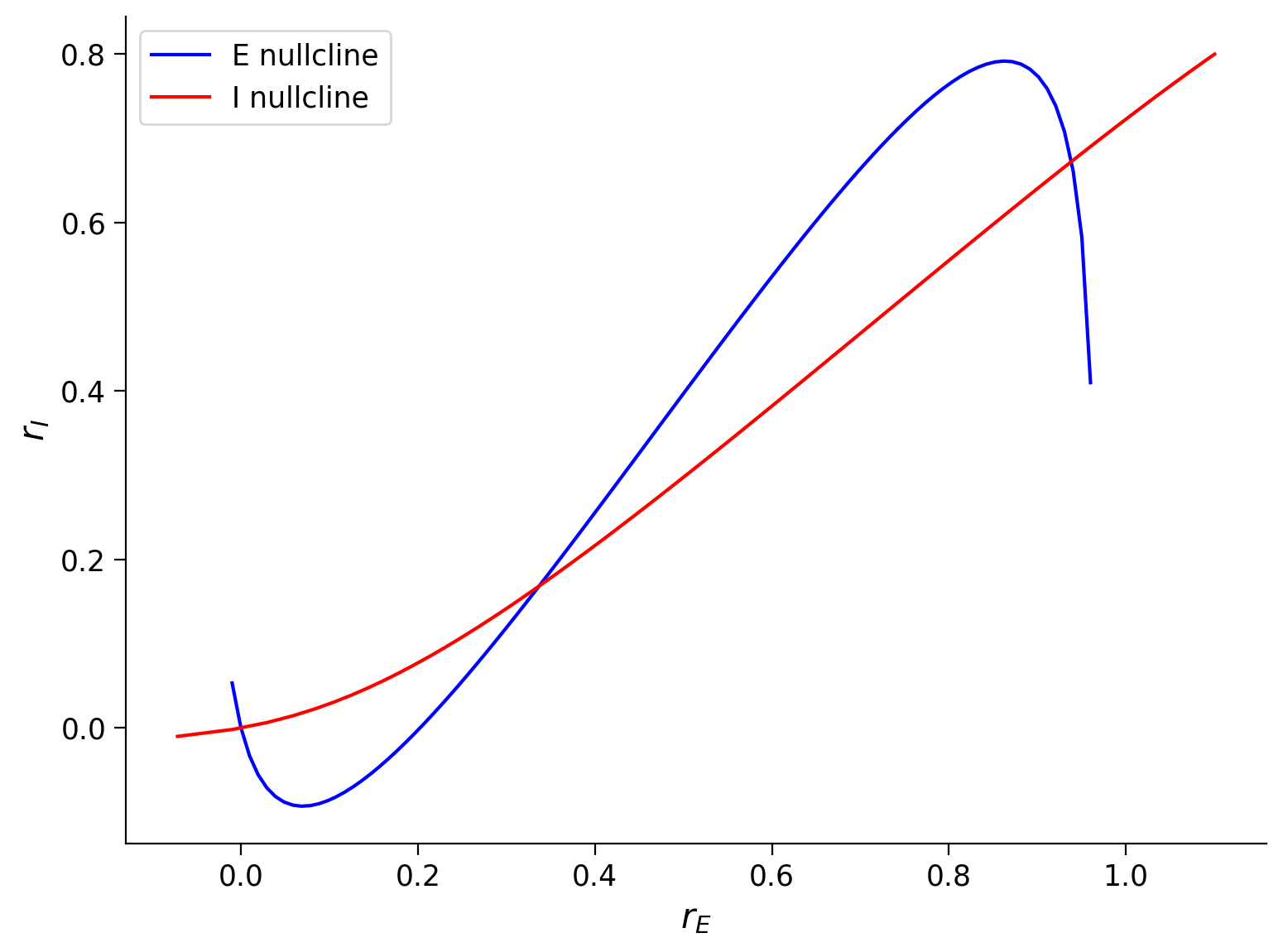
Execute the cell to define my_fp and check_fp
Show code cell source
# @markdown *Execute the cell to define `my_fp` and `check_fp`*
def my_fp(pars, rE_init, rI_init):
"""
Use opt.root function to solve Equations (2)-(3) from initial values
"""
tau_E, a_E, theta_E = pars['tau_E'], pars['a_E'], pars['theta_E']
tau_I, a_I, theta_I = pars['tau_I'], pars['a_I'], pars['theta_I']
wEE, wEI = pars['wEE'], pars['wEI']
wIE, wII = pars['wIE'], pars['wII']
I_ext_E, I_ext_I = pars['I_ext_E'], pars['I_ext_I']
# define the right hand of wilson-cowan equations
def my_WCr(x):
rE, rI = x
drEdt = (-rE + F(wEE * rE - wEI * rI + I_ext_E, a_E, theta_E)) / tau_E
drIdt = (-rI + F(wIE * rE - wII * rI + I_ext_I, a_I, theta_I)) / tau_I
y = np.array([drEdt, drIdt])
return y
x0 = np.array([rE_init, rI_init])
x_fp = opt.root(my_WCr, x0).x
return x_fp
def check_fp(pars, x_fp, mytol=1e-6):
"""
Verify (drE/dt)^2 + (drI/dt)^2< mytol
Args:
pars : Parameter dictionary
fp : value of fixed point
mytol : tolerance, default as 10^{-6}
Returns :
Whether it is a correct fixed point: True/False
"""
drEdt, drIdt = EIderivs(x_fp[0], x_fp[1], **pars)
return drEdt**2 + drIdt**2 < mytol
help(my_fp)
Help on function my_fp in module __main__:
my_fp(pars, rE_init, rI_init)
Use opt.root function to solve Equations (2)-(3) from initial values
Coding Exercise 1.1: Find the fixed points of the Wilson-Cowan model#
From the above nullclines, we notice that the system features three fixed points with the parameters we used. To find their coordinates, we need to choose a proper initial value to give to the opt.root function inside of the function my_fp we just defined, since the algorithm can only find fixed points in the vicinity of the initial value.
In this exercise, you will use the function my_fp to find each of the fixed points by varying the initial values. Note that you can choose the values near the intersections of the nullclines as the initial values to calculate the fixed points.
pars = default_pars()
######################################################################
# TODO: Provide initial values to calculate the fixed points
# Check if x_fp's are the correct with the function check_fp(x_fp)
# Hint: vary different initial values to find the correct fixed points
raise NotImplementedError('student exercise: find fixed points')
######################################################################
my_plot_nullcline(pars)
# Find the first fixed point
x_fp_1 = my_fp(pars, ..., ...)
if check_fp(pars, x_fp_1):
plot_fp(x_fp_1)
# Find the second fixed point
x_fp_2 = my_fp(pars, ..., ...)
if check_fp(pars, x_fp_2):
plot_fp(x_fp_2)
# Find the third fixed point
x_fp_3 = my_fp(pars, ..., ...)
if check_fp(pars, x_fp_3):
plot_fp(x_fp_3)
Example output:
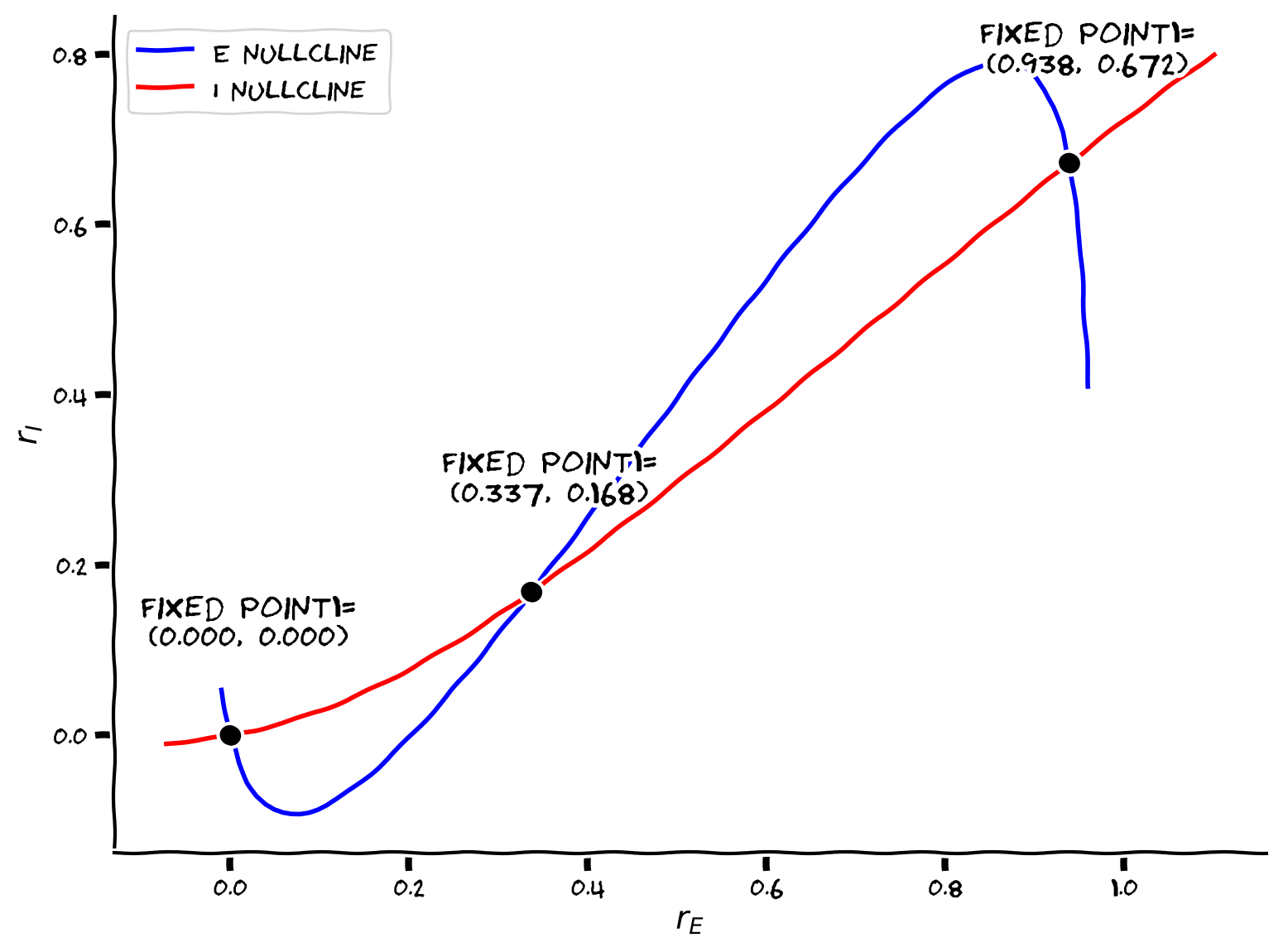
Submit your feedback#
Show code cell source
# @title Submit your feedback
content_review(f"{feedback_prefix}_Find_the_Fixed_points_of_WE_Exercise")
Section 1.2: Stability of a fixed point and eigenvalues of the Jacobian Matrix#
First, let’s first rewrite the system \(1\) as:
where
By definition, \(\displaystyle\frac{dr_E}{dt}=0\) and \(\displaystyle\frac{dr_I}{dt}=0\) at each fixed point. Therefore, if the initial state is exactly at the fixed point, the state of the system will not change as time evolves.
However, if the initial state deviates slightly from the fixed point, there are two possibilities the trajectory will be attracted back to the
The trajectory will be attracted back to the fixed point
The trajectory will diverge from the fixed point.
These two possibilities define the type of fixed point, i.e., stable or unstable. Similar to the 1D system studied in the previous tutorial, the stability of a fixed point \((r_E^*, r_I^*)\) can be determined by linearizing the dynamics of the system (can you figure out how?). The linearization will yield a matrix of first-order derivatives called the Jacobian matrix:
The eigenvalues of the Jacobian matrix calculated at the fixed point will determine whether it is a stable or unstable fixed point.
We can now compute the derivatives needed to build the Jacobian matrix. Using the chain and product rules the derivatives for the excitatory population are given by:
The same applies to the inhibitory population.
Coding Exercise 1.2: Compute the Jacobian Matrix for the Wilson-Cowan model#
Here, you can use dF(x,a,theta) defined in the Helper functions to calculate the derivative of the F-I curve.
def get_eig_Jacobian(fp,
tau_E, a_E, theta_E, wEE, wEI, I_ext_E,
tau_I, a_I, theta_I, wIE, wII, I_ext_I, **other_pars):
"""Compute eigenvalues of the Wilson-Cowan Jacobian matrix at fixed point."""
# Initialization
rE, rI = fp
J = np.zeros((2, 2))
###########################################################################
# TODO for students: compute J and disable the error
raise NotImplementedError("Student exercise: compute the Jacobian matrix")
###########################################################################
# Compute the four elements of the Jacobian matrix
J[0, 0] = ...
J[0, 1] = ...
J[1, 0] = ...
J[1, 1] = ...
# Compute and return the eigenvalues
evals = np.linalg.eig(J)[0]
return evals
# Compute eigenvalues of Jacobian
eig_1 = get_eig_Jacobian(x_fp_1, **pars)
eig_2 = get_eig_Jacobian(x_fp_2, **pars)
eig_3 = get_eig_Jacobian(x_fp_3, **pars)
print(eig_1, 'Stable point')
print(eig_2, 'Unstable point')
print(eig_3, 'Stable point')
Submit your feedback#
Show code cell source
# @title Submit your feedback
content_review(f"{feedback_prefix}_Compute_the_Jacobian_Exercise")
As is evident, the stable fixed points correspond to the negative eigenvalues, while unstable points correspond to at least one positive eigenvalue.
The sign of the eigenvalues is determined by the connectivity (interaction) between excitatory and inhibitory populations.
Below we investigate the effect of \(w_{EE}\) on the nullclines and the eigenvalues of the dynamical system.
* Critical change is referred to as pitchfork bifurcation.
Section 1.3: Effect of wEE on the nullclines and the eigenvalues#
Interactive Demo 1.3: Nullclines position in the phase plane changes with parameter values#
How do the nullclines move for different values of the parameter \(w_{EE}\)? What does this mean for fixed points and system activity?
Make sure you execute this cell to enable the widget!
Show code cell source
# @markdown Make sure you execute this cell to enable the widget!
@widgets.interact(
wEE=widgets.FloatSlider(6., min=6., max=10., step=0.01)
)
def plot_nullcline_diffwEE(wEE):
"""
plot nullclines for different values of wEE
"""
pars = default_pars(wEE=wEE)
# plot the E, I nullclines
Exc_null_rE = np.linspace(-0.01, .96, 100)
Exc_null_rI = get_E_nullcline(Exc_null_rE, **pars)
Inh_null_rI = np.linspace(-.01, .8, 100)
Inh_null_rE = get_I_nullcline(Inh_null_rI, **pars)
plt.figure(figsize=(12, 5.5))
plt.subplot(121)
plt.plot(Exc_null_rE, Exc_null_rI, 'b', label='E nullcline')
plt.plot(Inh_null_rE, Inh_null_rI, 'r', label='I nullcline')
plt.xlabel(r'$r_E$')
plt.ylabel(r'$r_I$')
plt.legend(loc='best')
plt.subplot(222)
pars['rE_init'], pars['rI_init'] = 0.2, 0.2
rE, rI = simulate_wc(**pars)
plt.plot(pars['range_t'], rE, 'b', label='E population', clip_on=False)
plt.plot(pars['range_t'], rI, 'r', label='I population', clip_on=False)
plt.ylabel('Activity')
plt.legend(loc='best')
plt.ylim(-0.05, 1.05)
plt.title('E/I activity\nfor different initial conditions',
fontweight='bold')
plt.subplot(224)
pars['rE_init'], pars['rI_init'] = 0.4, 0.1
rE, rI = simulate_wc(**pars)
plt.plot(pars['range_t'], rE, 'b', label='E population', clip_on=False)
plt.plot(pars['range_t'], rI, 'r', label='I population', clip_on=False)
plt.xlabel('t (ms)')
plt.ylabel('Activity')
plt.legend(loc='best')
plt.ylim(-0.05, 1.05)
plt.tight_layout()
plt.show()
Submit your feedback#
Show code cell source
# @title Submit your feedback
content_review(f"{feedback_prefix}_Effect_of_wEE_Interactive_Demo_and_Discussion")
We can also investigate the effect of different \(w_{EI}\), \(w_{IE}\), \(w_{II}\), \(\tau_{E}\), \(\tau_{I}\), and \(I_{E}^{\text{ext}}\) on the stability of fixed points. In addition, we can also consider the perturbation of the parameters of the gain curve \(F(\cdot)\).
Section 1.4: Limit cycle - Oscillations#
For some values of interaction terms (\(w_{EE}, w_{IE}, w_{EI}, w_{II}\)), the eigenvalues can become complex. When at least one pair of eigenvalues is complex, oscillations arise. The stability of oscillations is determined by the real part of the eigenvalues (+ve real part oscillations will grow, -ve real part oscillations will die out). The size of the complex part determines the frequency of oscillations.
For instance, if we use a different set of parameters, \(w_{EE}=6.4\), \(w_{EI}=4.8\), \(w_{IE}=6.\), \(w_{II}=1.2\), and \(I_{E}^{\text{ext}}=0.8\), then we shall observe that the E and I population activity start to oscillate! Please execute the cell below to check the oscillatory behavior.
Make sure you execute this cell to see the oscillations!
Show code cell source
# @markdown Make sure you execute this cell to see the oscillations!
pars = default_pars(T=100.)
pars['wEE'], pars['wEI'] = 6.4, 4.8
pars['wIE'], pars['wII'] = 6.0, 1.2
pars['I_ext_E'] = 0.8
pars['rE_init'], pars['rI_init'] = 0.25, 0.25
rE, rI = simulate_wc(**pars)
plt.figure(figsize=(8, 5.5))
plt.plot(pars['range_t'], rE, 'b', label=r'$r_E$')
plt.plot(pars['range_t'], rI, 'r', label=r'$r_I$')
plt.xlabel('t (ms)')
plt.ylabel('Activity')
plt.legend(loc='best')
plt.show()
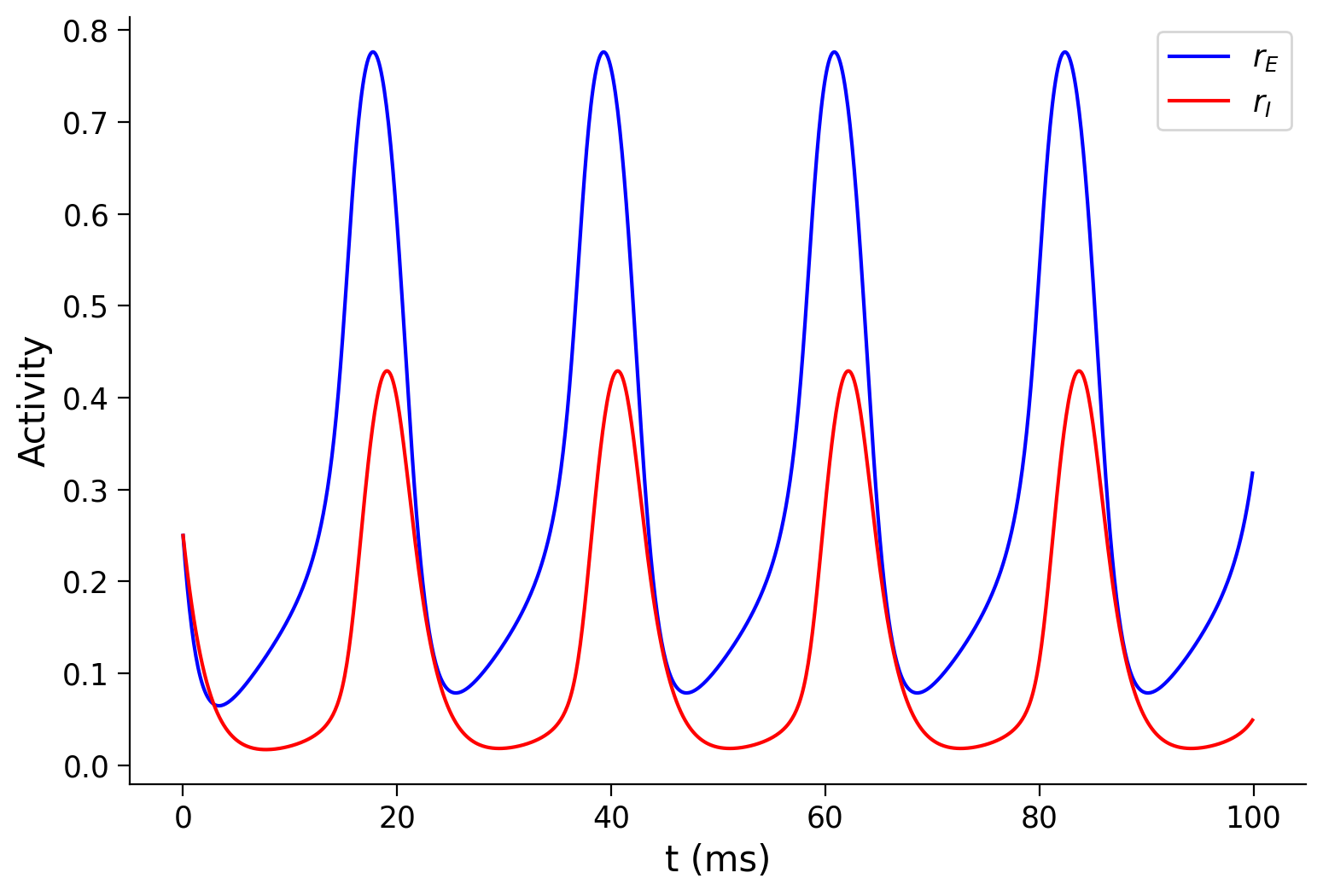
We can also understand the oscillations of the population behavior using the phase plane. By plotting a set of trajectories with different initial states, we can see that these trajectories will move in a circle instead of converging to a fixed point. This circle is called “limit cycle” and shows the periodic oscillations of the \(E\) and \(I\) population behavior under some conditions.
Let’s plot the phase plane using the previously defined functions.
Execute to visualize phase plane
Show code cell source
# @markdown Execute to visualize phase plane
pars = default_pars(T=100.)
pars['wEE'], pars['wEI'] = 6.4, 4.8
pars['wIE'], pars['wII'] = 6.0, 1.2
pars['I_ext_E'] = 0.8
plt.figure(figsize=(7, 5.5))
my_plot_nullcline(pars)
# Find the correct fixed point
x_fp_1 = my_fp(pars, 0.8, 0.8)
if check_fp(pars, x_fp_1):
plot_fp(x_fp_1, position=(0, 0), rotation=40)
my_plot_trajectories(pars, 0.2, 3,
'Sample trajectories \nwith different initial values')
my_plot_vector(pars)
plt.legend(loc=[1.01, 0.7])
plt.xlim(-0.05, 1.01)
plt.ylim(-0.05, 0.65)
plt.show()
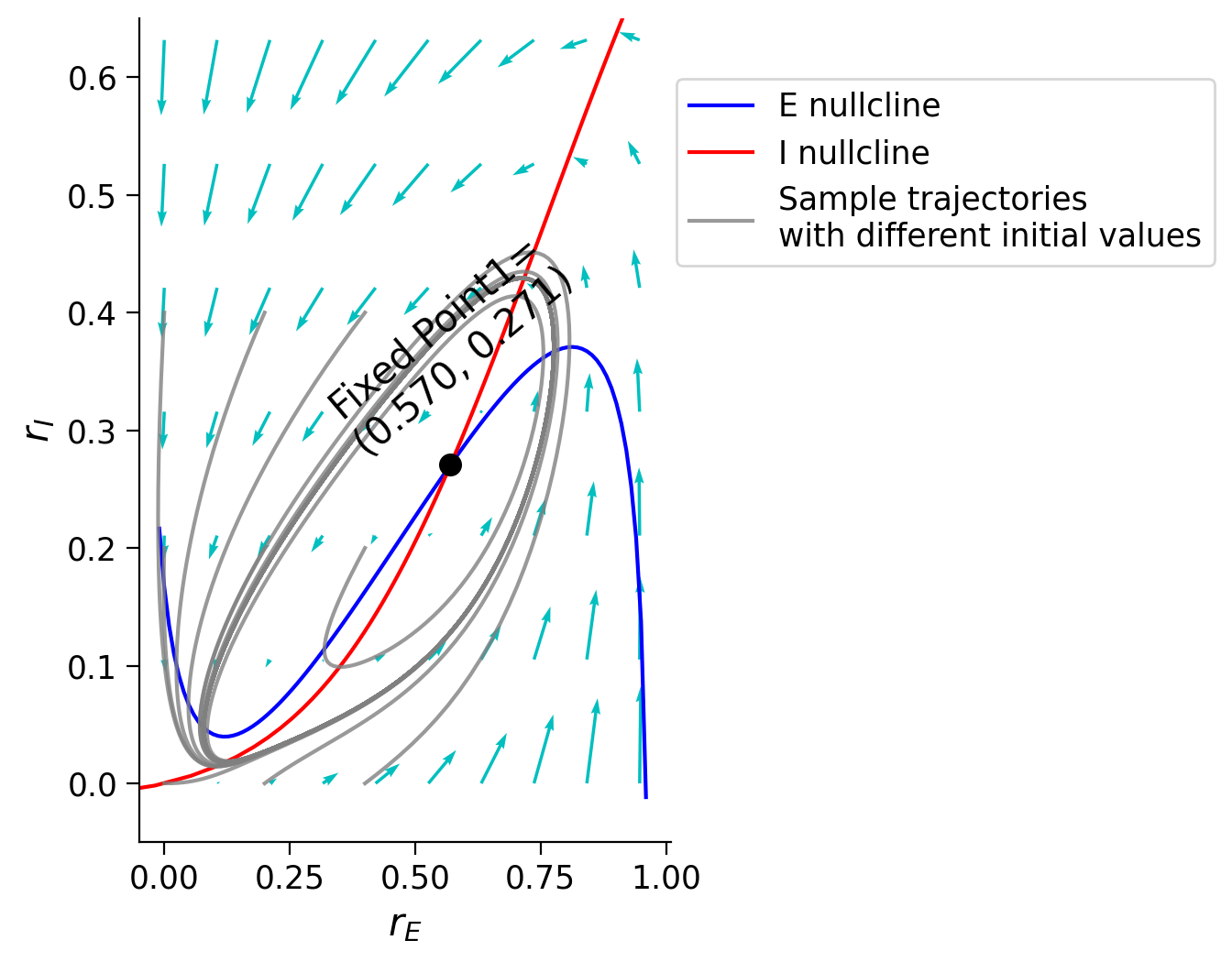
Interactive Demo 1.4: Limit cycle and oscillations#
From the above examples, the change of model parameters changes the shape of the nullclines and, accordingly, the behavior of the \(E\) and \(I\) populations from steady fixed points to oscillations. However, the shape of the nullclines is unable to fully determine the behavior of the network. The vector field also matters. To demonstrate this, here, we will investigate the effect of time constants on the population behavior. By changing the inhibitory time constant \(\tau_I\), the nullclines do not change, but the network behavior changes substantially from steady state to oscillations with different frequencies.
Such a dramatic change in the system behavior is referred to as a bifurcation.
Please execute the code below to check this out.
Make sure you execute this cell to enable the widget!
Show code cell source
# @markdown Make sure you execute this cell to enable the widget!
@widgets.interact(
tau_i=widgets.FloatSlider(1.5, min=0.2, max=3., step=.1)
)
def time_constant_effect(tau_i=0.5):
pars = default_pars(T=100.)
pars['wEE'], pars['wEI'] = 6.4, 4.8
pars['wIE'], pars['wII'] = 6.0, 1.2
pars['I_ext_E'] = 0.8
pars['tau_I'] = tau_i
Exc_null_rE = np.linspace(0.0, .9, 100)
Inh_null_rI = np.linspace(0.0, .6, 100)
Exc_null_rI = get_E_nullcline(Exc_null_rE, **pars)
Inh_null_rE = get_I_nullcline(Inh_null_rI, **pars)
plt.figure(figsize=(12.5, 5.5))
plt.subplot(121) # nullclines
plt.plot(Exc_null_rE, Exc_null_rI, 'b', label='E nullcline', zorder=2)
plt.plot(Inh_null_rE, Inh_null_rI, 'r', label='I nullcline', zorder=2)
plt.xlabel(r'$r_E$')
plt.ylabel(r'$r_I$')
# fixed point
x_fp_1 = my_fp(pars, 0.5, 0.5)
plt.plot(x_fp_1[0], x_fp_1[1], 'ko', zorder=2)
eig_1 = get_eig_Jacobian(x_fp_1, **pars)
# trajectories
for ie in range(5):
for ii in range(5):
pars['rE_init'], pars['rI_init'] = 0.1 * ie, 0.1 * ii
rE_tj, rI_tj = simulate_wc(**pars)
plt.plot(rE_tj, rI_tj, 'k', alpha=0.3, zorder=1)
# vector field
EI_grid_E = np.linspace(0., 1.0, 20)
EI_grid_I = np.linspace(0., 0.6, 20)
rE, rI = np.meshgrid(EI_grid_E, EI_grid_I)
drEdt, drIdt = EIderivs(rE, rI, **pars)
n_skip = 2
plt.quiver(rE[::n_skip, ::n_skip], rI[::n_skip, ::n_skip],
drEdt[::n_skip, ::n_skip], drIdt[::n_skip, ::n_skip],
angles='xy', scale_units='xy', scale=10, facecolor='c')
plt.title(r'$\tau_I=$'+'%.1f ms' % tau_i)
plt.subplot(122) # sample E/I trajectories
pars['rE_init'], pars['rI_init'] = 0.25, 0.25
rE, rI = simulate_wc(**pars)
plt.plot(pars['range_t'], rE, 'b', label=r'$r_E$')
plt.plot(pars['range_t'], rI, 'r', label=r'$r_I$')
plt.xlabel('t (ms)')
plt.ylabel('Activity')
plt.title(r'$\tau_I=$'+'%.1f ms' % tau_i)
plt.legend(loc='best')
plt.tight_layout()
plt.show()
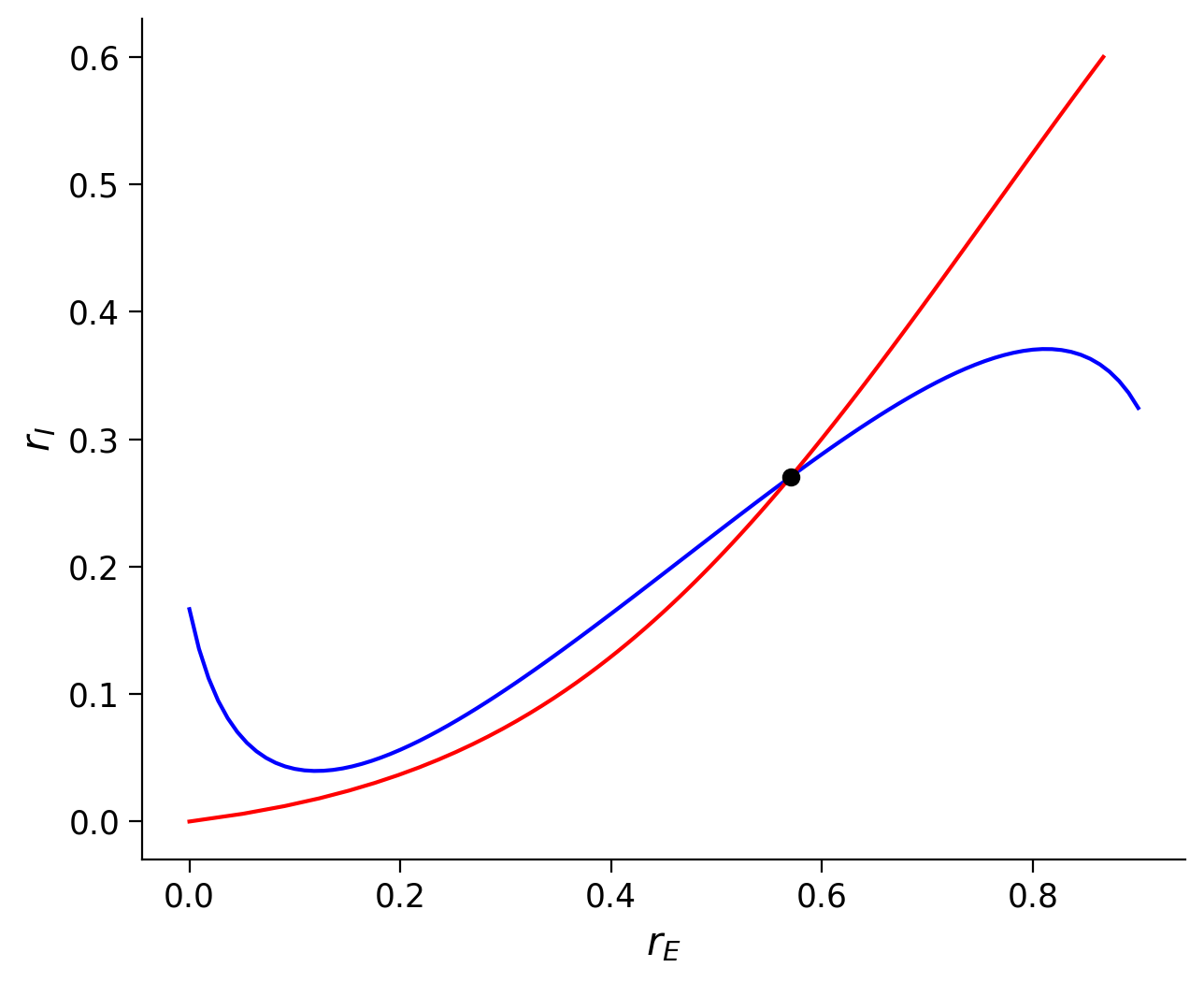
Both \(\tau_E\) and \(\tau_I\) feature in the Jacobian of the two population network (eq 7). So here is seems that the by increasing \(\tau_I\) the eigenvalues corresponding to the stable fixed point are becoming complex.
Intuitively, when \(\tau_I\) is smaller, inhibitory activity changes faster than excitatory activity. As inhibition exceeds above a certain value, high inhibition inhibits excitatory population but that in turns means that inhibitory population gets smaller input (from the exc. connection). So inhibition decreases rapidly. But this means that excitation recovers – and so on …
Submit your feedback#
Show code cell source
# @title Submit your feedback
content_review(f"{feedback_prefix}_Limit_cycle_and_oscillations_Interactive_Demo")
Section 2: Inhibition-stabilized network (ISN)#
Section 2.1: Inhibition-stabilized network#
As described above, one can obtain the linear approximation around the fixed point as
where \(\vec{R} = [r_E, r_I]^{\rm T}\) is the vector of the E/I activity.
Let’s direct our attention to the excitatory subpopulation which follows:
Recall that, around fixed point \((r_E^*, r_I^*)\):
From Equation. (8), it is clear that \(\displaystyle{\frac{\partial G_E}{\partial r_I}}\) is negative since the \(\displaystyle{\frac{dF}{dx}}\) is always positive. It can be understood by that the recurrent inhibition from the inhibitory activity (\(I\)) can reduce the excitatory (\(E\)) activity. However, as described above, \(\displaystyle{\frac{\partial G_E}{\partial r_E}}\) has negative terms related to the “leak” effect, and positive terms related to the recurrent excitation. Therefore, it leads to two different regimes:
\(\displaystyle{\frac{\partial}{\partial r_E}G_E(r_E^*, r_I^*)}<0\), noninhibition-stabilized network (non-ISN) regime
\(\displaystyle{\frac{\partial}{\partial r_E}G_E(r_E^*, r_I^*)}>0\), inhibition-stabilized network (ISN) regime
Coding Exercise 2.1: Compute \(\displaystyle{\frac{\partial G_E}{\partial r_E}}\)#
Implement the function to calculate the \(\displaystyle{\frac{\partial G_E}{\partial r_E}}\) for the default parameters, and the parameters of the limit cycle case.
def get_dGdE(fp, tau_E, a_E, theta_E, wEE, wEI, I_ext_E, **other_pars):
"""
Compute dGdE
Args:
fp : fixed point (E, I), array
Other arguments are parameters of the Wilson-Cowan model
Returns:
J : the 2x2 Jacobian matrix
"""
rE, rI = fp
##########################################################################
# TODO for students: compute dGdrE and disable the error
raise NotImplementedError("Student exercise: compute the dG/dE, Eq. (13)")
##########################################################################
# Calculate the J[0,0]
dGdrE = ...
return dGdrE
# Get fixed points
pars = default_pars()
x_fp_1 = my_fp(pars, 0.1, 0.1)
x_fp_2 = my_fp(pars, 0.3, 0.3)
x_fp_3 = my_fp(pars, 0.8, 0.6)
# Compute dGdE
dGdrE1 = get_dGdE(x_fp_1, **pars)
dGdrE2 = get_dGdE(x_fp_2, **pars)
dGdrE3 = get_dGdE(x_fp_3, **pars)
print(f'For the default case:')
print(f'dG/drE(fp1) = {dGdrE1:.3f}')
print(f'dG/drE(fp2) = {dGdrE2:.3f}')
print(f'dG/drE(fp3) = {dGdrE3:.3f}')
print('\n')
pars = default_pars(wEE=6.4, wEI=4.8, wIE=6.0, wII=1.2, I_ext_E=0.8)
x_fp_lc = my_fp(pars, 0.8, 0.8)
dGdrE_lc = get_dGdE(x_fp_lc, **pars)
print('For the limit cycle case:')
print(f'dG/drE(fp_lc) = {dGdrE_lc:.3f}')
SAMPLE OUTPUT
For the default case:
dG/drE(fp1) = -0.650
dG/drE(fp2) = 1.519
dG/drE(fp3) = -0.706
For the limit cycle case:
dG/drE(fp_lc) = 0.837
Submit your feedback#
Show code cell source
# @title Submit your feedback
content_review(f"{feedback_prefix}_Compute_dGdrE_Exercise")
Section 2.2: Nullcline analysis of the ISN#
Recall that the E nullcline follows
That is, the firing rate \(r_E\) can be a function of \(r_I\). Let’s take the derivative of \(r_E\) over \(r_I\), and obtain
That is, in the phase plane rI-rE-plane, we can obtain the slope along the E nullcline as
Similarly, we can obtain the slope along the I nullcline as
Then, we can find that \(\Big{(} \displaystyle{\frac{dr_I}{dr_E}} \Big{)}_{\rm I-nullcline} >0\) in Equation (13).
However, in Equation (12), the sign of \(\Big{(} \displaystyle{\frac{dr_I}{dr_E}} \Big{)}_{\rm E-nullcline}\) depends on the sign of \((F_E'w_{EE}-1)\). Note that, \((F_E'w_{EE}-1)\) is the same as what we show above (Equation (8)). Therefore, we can have the following results:
\(\Big{(} \displaystyle{\frac{dr_I}{dr_E}} \Big{)}_{\rm E-nullcline}<0\), noninhibition-stabilized network (non-ISN) regime
\(\Big{(} \displaystyle{\frac{dr_I}{dr_E}} \Big{)}_{\rm E-nullcline}>0\), inhibition-stabilized network (ISN) regime
In addition, it is important to point out the following two conclusions:
Conclusion 1: The stability of a fixed point can determine the relationship between the slopes Equations (12) and (13). As discussed above, the fixed point is stable when the Jacobian matrix (\(J\) in Equation (7)) has two eigenvalues with a negative real part, which indicates a positive determinant of \(J\), i.e., \(\text{det}(J)>0\).
From the Jacobian matrix definition and from Equations (8-11), we can obtain:
Note that, if we let
then, using matrix notation, \(J=T^{-1}(F W - I)\) where \(I\) is the identity matrix, i.e., \(I = \begin{bmatrix} 1 & 0 \\ 0 & 1 \end{bmatrix}.\)
Therefore, \(\det{(J)}=\det{(T^{-1}(F W - I))}=(\det{(T^{-1})})(\det{(F W - I)}).\)
Since \(\det{(T^{-1})}>0\), as time constants are positive by definition, the sign of \(\det{(J)}\) is the same as the sign of \(\det{(F W - I)}\), and so
Then, combining this with Equations (12) and (13), we can obtain
Therefore, at the stable fixed point, I nullcline has a steeper slope than the E nullcline.
Conclusion 2: Effect of adding input to the inhibitory population.
While adding the input \(\delta I^{\rm ext}_I\) into the inhibitory population, we can find that the E nullcline (Equation (5)) stays the same, while the I nullcline has a pure left shift: the original I nullcline equation,
remains true if we take \(I^{\text{ext}}_I \rightarrow I^{\text{ext}}_I +\delta I^{\rm ext}_I\) and \(r_E\rightarrow r_E'=r_E-\frac{\delta I^{\rm ext}_I}{w_{IE}}\) to obtain
Putting these points together, we obtain the phase plane pictures shown below. After adding input to the inhibitory population, it can be seen in the trajectories above and the phase plane below that, in an ISN, \(r_I\) will increase first but then decay to the new fixed point in which both \(r_I\) and \(r_E\) are decreased compared to the original fixed point. However, by adding \(\delta I^{\rm ext}_I\) into a non-ISN, \(r_I\) will increase while \(r_E\) will decrease.
Interactive Demo 2.2: Nullclines of Example ISN and non-ISN#
In this interactive widget, we inject excitatory (\(I^{\text{ext}}_I>0\)) or inhibitory (\(I^{\text{ext}}_I<0\)) drive into the inhibitory population when the system is at its equilibrium (with parameters \(w_{EE}=6.4\), \(w_{EI}=4.8\), \(w_{IE}=6.\), \(w_{II}=1.2\), \(I_{E}^{\text{ext}}=0.8\), \(\tau_I = 0.8\), and \(I^{\text{ext}}_I=0\)). How does the firing rate of the \(I\) population changes with excitatory vs inhibitory drive into the inhibitory population?
#
Make sure you execute this cell to enable the widget!
Show code cell source
# @title
# @markdown Make sure you execute this cell to enable the widget!
pars = default_pars(T=50., dt=0.1)
pars['wEE'], pars['wEI'] = 6.4, 4.8
pars['wIE'], pars['wII'] = 6.0, 1.2
pars['I_ext_E'] = 0.8
pars['tau_I'] = 0.8
@widgets.interact(
dI=widgets.FloatSlider(0., min=-0.2, max=0.2, step=.05)
)
def ISN_I_perturb(dI=0.1):
Lt = len(pars['range_t'])
pars['I_ext_I'] = np.zeros(Lt)
pars['I_ext_I'][int(Lt / 2):] = dI
pars['rE_init'], pars['rI_init'] = 0.6, 0.26
rE, rI = simulate_wc(**pars)
plt.figure(figsize=(8, 1.5))
plt.plot(pars['range_t'], pars['I_ext_I'], 'k')
plt.xlabel('t (ms)')
plt.ylabel(r'$I_I^{\mathrm{ext}}$')
plt.ylim(pars['I_ext_I'].min() - 0.01, pars['I_ext_I'].max() + 0.01)
plt.show()
plt.figure(figsize=(8, 4.5))
plt.plot(pars['range_t'], rE, 'b', label=r'$r_E$')
plt.plot(pars['range_t'], rE[int(Lt / 2) - 1] * np.ones(Lt), 'b--')
plt.plot(pars['range_t'], rI, 'r', label=r'$r_I$')
plt.plot(pars['range_t'], rI[int(Lt / 2) - 1] * np.ones(Lt), 'r--')
plt.ylim(0, 0.8)
plt.xlabel('t (ms)')
plt.ylabel('Activity')
plt.legend(loc='best')
plt.show()
Submit your feedback#
Show code cell source
# @title Submit your feedback
content_review(f"{feedback_prefix}_Nullclines_of_ISN_and_nonISN_Interactive_Demo_and_Discussion")
Section 3: Fixed point and working memory#
The input into the neurons measured in the experiment is often very noisy (links). Here, the noisy synaptic input current is modeled as an Ornstein-Uhlenbeck (OU)process, which has been discussed several times in the previous tutorials.
Make sure you execute this cell to enable the function my_OU and plot the input current!
Show code cell source
# @markdown Make sure you execute this cell to enable the function my_OU and plot the input current!
def my_OU(pars, sig, myseed=False):
"""
Expects:
pars : parameter dictionary
sig : noise amplitute
myseed : random seed. int or boolean
Returns:
I : Ornstein-Uhlenbeck input current
"""
# Retrieve simulation parameters
dt, range_t = pars['dt'], pars['range_t']
Lt = range_t.size
tau_ou = pars['tau_ou'] # [ms]
# set random seed
if myseed:
np.random.seed(seed=myseed)
else:
np.random.seed()
# Initialize
noise = np.random.randn(Lt)
I_ou = np.zeros(Lt)
I_ou[0] = noise[0] * sig
# generate OU
for it in range(Lt-1):
I_ou[it+1] = (I_ou[it]
+ dt / tau_ou * (0. - I_ou[it])
+ np.sqrt(2 * dt / tau_ou) * sig * noise[it + 1])
return I_ou
pars = default_pars(T=50)
pars['tau_ou'] = 1. # [ms]
sig_ou = 0.1
I_ou = my_OU(pars, sig=sig_ou, myseed=2020)
plt.figure(figsize=(8, 5.5))
plt.plot(pars['range_t'], I_ou, 'b')
plt.xlabel('Time (ms)')
plt.ylabel(r'$I_{\mathrm{OU}}$')
plt.show()
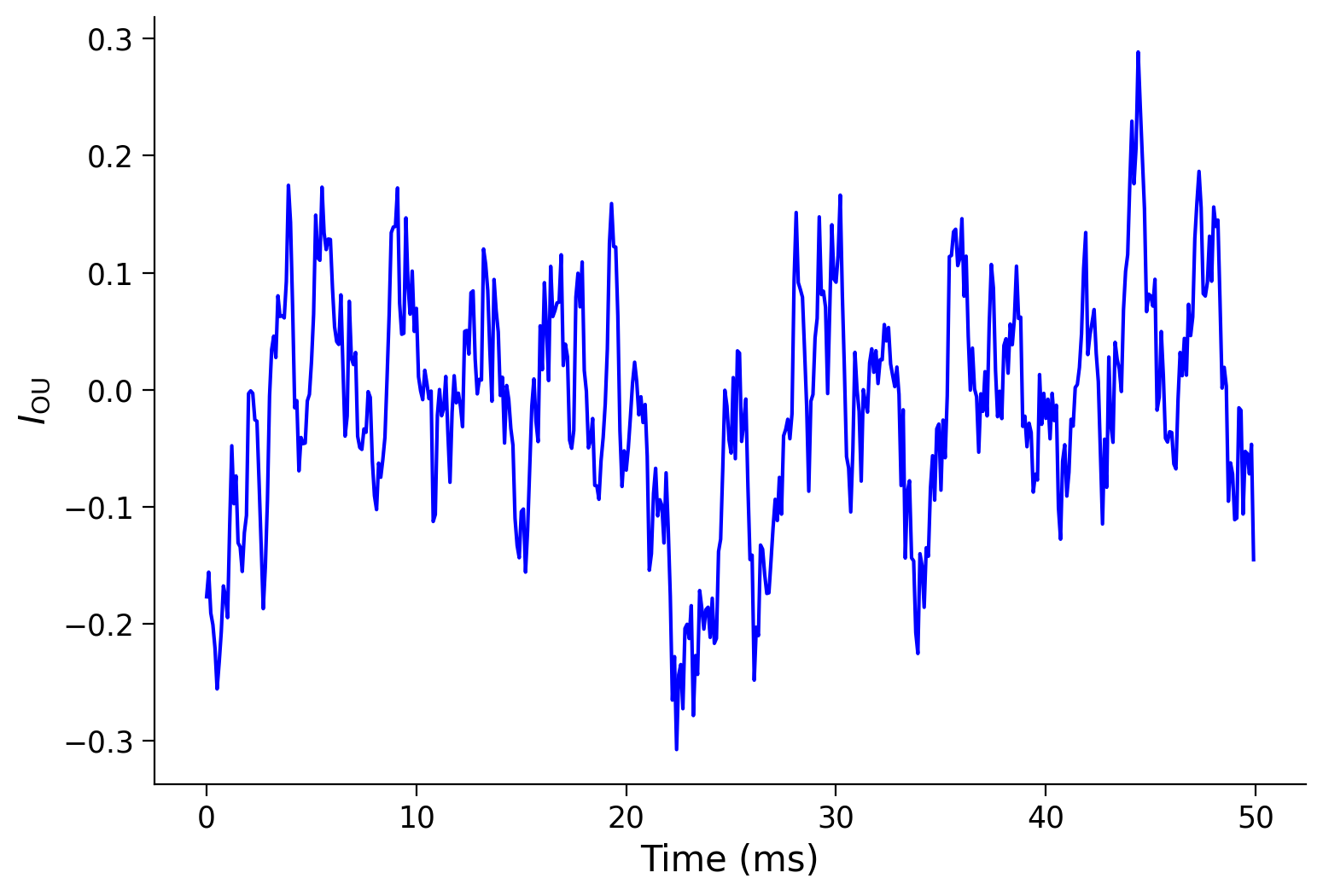
With the default parameters, the system fluctuates around a resting state with the noisy input.
Execute this cell to plot activity with noisy input current
Show code cell source
# @markdown Execute this cell to plot activity with noisy input current
pars = default_pars(T=100)
pars['tau_ou'] = 1. # [ms]
sig_ou = 0.1
pars['I_ext_E'] = my_OU(pars, sig=sig_ou, myseed=20201)
pars['I_ext_I'] = my_OU(pars, sig=sig_ou, myseed=20202)
pars['rE_init'], pars['rI_init'] = 0.1, 0.1
rE, rI = simulate_wc(**pars)
plt.figure(figsize=(8, 5.5))
ax = plt.subplot(111)
ax.plot(pars['range_t'], rE, 'b', label='E population')
ax.plot(pars['range_t'], rI, 'r', label='I population')
ax.set_xlabel('t (ms)')
ax.set_ylabel('Activity')
ax.legend(loc='best')
plt.show()
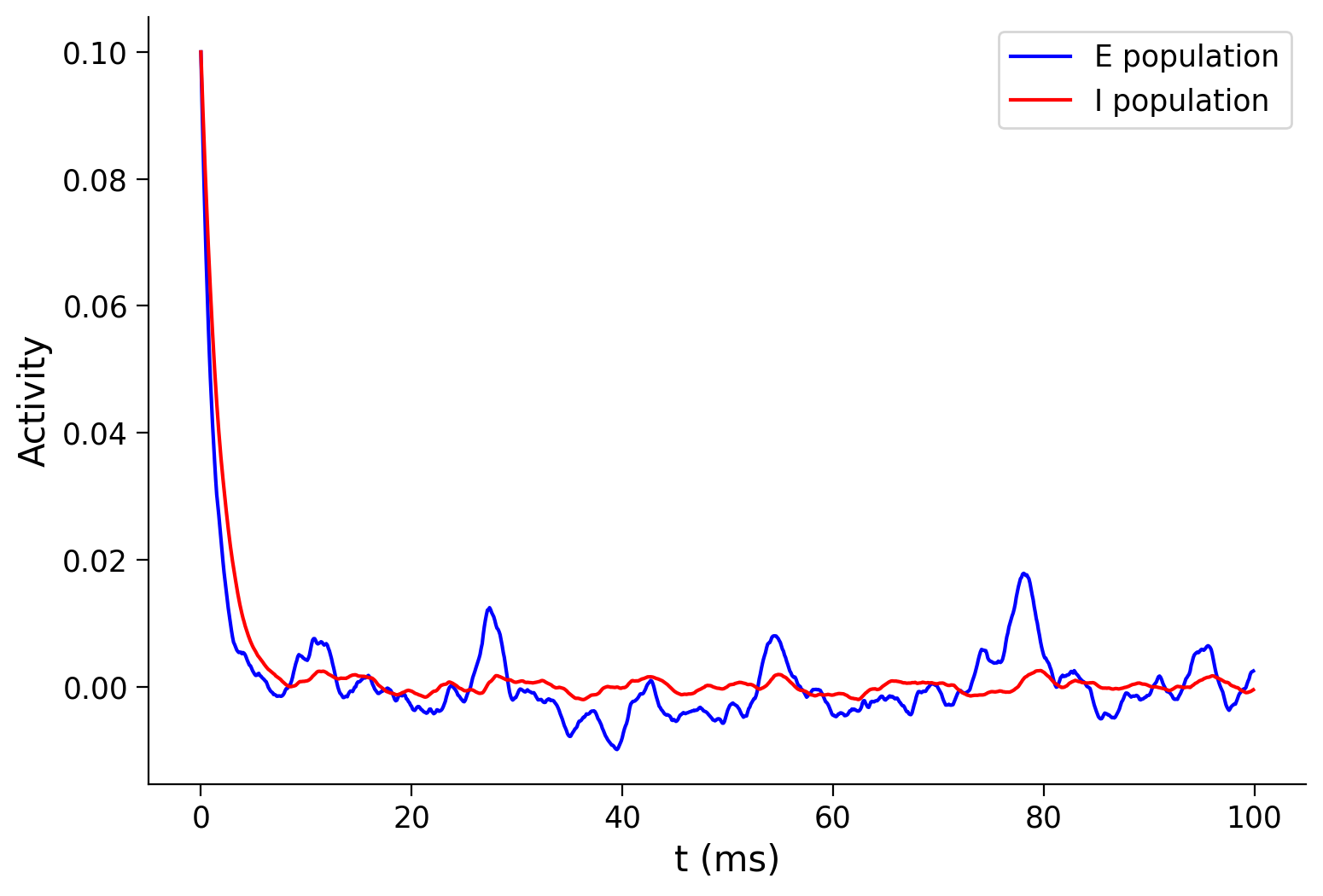
Interactive Demo 3: Short pulse induced persistent activity#
Then, let’s use a brief 10-ms positive current to the E population when the system is at its equilibrium. When this amplitude (SE below) is sufficiently large, a persistent activity is produced that outlasts the transient input. What is the firing rate of the persistent activity, and what is the critical input strength? Try to understand the phenomena from the above phase-plane analysis.
Make sure you execute this cell to enable the widget!
Show code cell source
# @markdown Make sure you execute this cell to enable the widget!
def my_inject(pars, t_start, t_lag=10.):
"""
Expects:
pars : parameter dictionary
t_start : pulse starts [ms]
t_lag : pulse lasts [ms]
Returns:
I : extra pulse time
"""
# Retrieve simulation parameters
dt, range_t = pars['dt'], pars['range_t']
Lt = range_t.size
# Initialize
I = np.zeros(Lt)
# pulse timing
N_start = int(t_start / dt)
N_lag = int(t_lag / dt)
I[N_start:N_start + N_lag] = 1.
return I
pars = default_pars(T=100)
pars['tau_ou'] = 1. # [ms]
sig_ou = 0.1
pars['I_ext_I'] = my_OU(pars, sig=sig_ou, myseed=2021)
pars['rE_init'], pars['rI_init'] = 0.1, 0.1
# pulse
I_pulse = my_inject(pars, t_start=20., t_lag=10.)
L_pulse = sum(I_pulse > 0.)
@widgets.interact(
SE=widgets.FloatSlider(0., min=0., max=1., step=.05)
)
def WC_with_pulse(SE=0.):
pars['I_ext_E'] = my_OU(pars, sig=sig_ou, myseed=2022)
pars['I_ext_E'] += SE * I_pulse
rE, rI = simulate_wc(**pars)
plt.figure(figsize=(8, 5.5))
ax = plt.subplot(111)
ax.plot(pars['range_t'], rE, 'b', label='E population')
ax.plot(pars['range_t'], rI, 'r', label='I population')
ax.plot(pars['range_t'][I_pulse > 0.], 1.0*np.ones(L_pulse), 'r', lw=3.)
ax.text(25, 1.05, 'stimulus on', horizontalalignment='center',
verticalalignment='bottom')
ax.set_ylim(-0.03, 1.2)
ax.set_xlabel('t (ms)')
ax.set_ylabel('Activity')
ax.legend(loc='best')
plt.show()
Submit your feedback#
Show code cell source
# @title Submit your feedback
content_review(f"{feedback_prefix}_Persistent_activity_Interactive_Demo_and_Discussion")
Explore what happens when a second, brief current is applied to the inhibitory population.

